Fluorine-containing functional diamine monomer with large conjugated structure as well as synthesis method and application thereof
A technology of conjugated structure and diamine monomer, which is applied in the field of material science, can solve the problems affecting the heat resistance, glass transition temperature and dimensional stability of polyimide materials, the performance needs to be further improved, and the variety is single. , to achieve obvious aggregation-induced luminescent performance, suitable for industrial production, and weaken the interaction force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
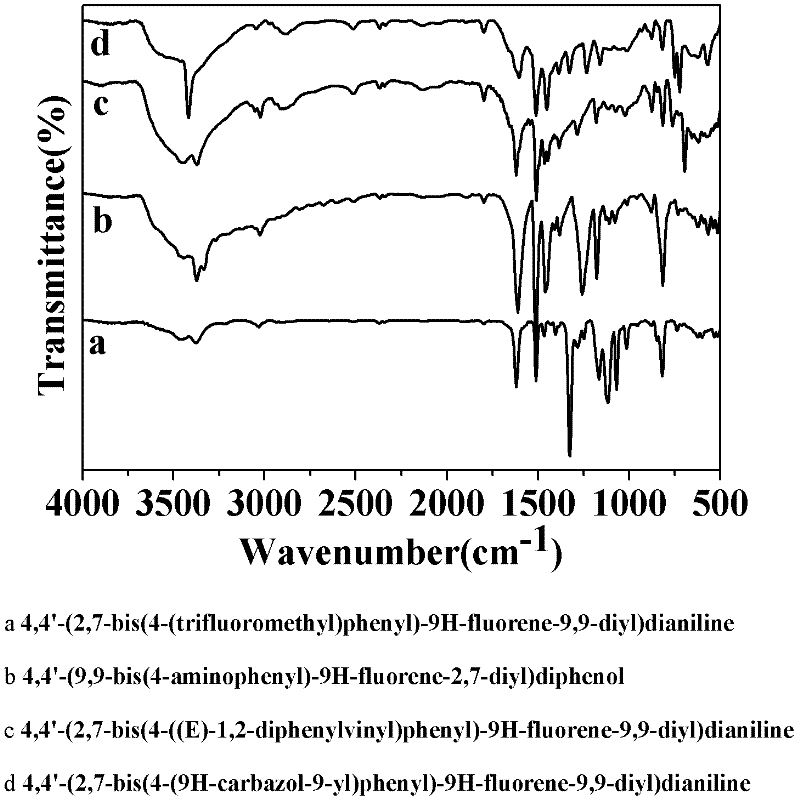
[0044] Synthesis of 4,4'-(2,7-bis(4-(trifluoromethyl)phenyl)-9H-fluorene-9,9-diyl)dianiline:
[0045]
[0046] (1) Synthesis of intermediate 2,7-dibromo-9,9-bis(4-nitrophenyl)-9H-fluorene:
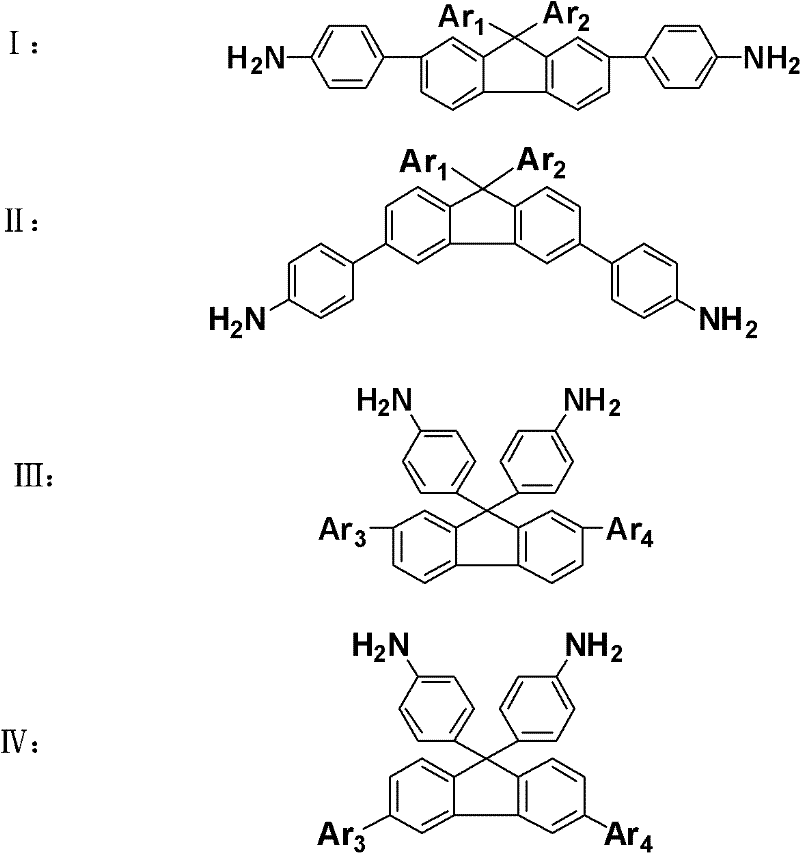
[0047] 32.401g (0.1mol) of 2,7-dibromofluorene (2,7-dibromo-9H-fluorene), 11.2g (0.1mol) of potassium tert-butoxide (t-BuOK) and 42.33g (0.3mol) of Add fluoronitrobenzene (1-fluoro-4-nitrobenzene) into a 500ml three-necked flask, use N,N-dimethylformamide (DMF) as a solvent, stir magnetically and pass argon gas, heat and reflux in an oil bath at 110°C for 24h , Pour the reaction solution into ice water, let it stand for 3h, and obtain a yellow solid by suction filtration, dissolve the yellow solid in absolute ethanol, and then crystallize at low temperature, filter the crystallized solid with suction to obtain a light yellow crystal, and in vacuo at 60°C After drying for 10 h, 51.5 g of the light yellow intermediate product 2,7-dibromo-9,9-bis(4-nitrophenyl)-9H-fluorene was obtained, w...
Embodiment 2
[0055] Synthesis of 4,4'-(9,9-bis(4-aminophenyl)-9H-fluorene-2,7-diyl)diphenol:
[0056]
[0057] (1) Synthesize intermediate 2,7-dibromo-9,9-bis(4-nitrophenyl)-9H-fluorene according to Example 1;
[0058] (2) Synthesis of intermediate 4,4'-(9,9-bis(4-nitrophenyl)-9H-fluorene-2,7-diyl)diphenol:
[0059] Add 11.124g (0.02mol) 2,7-dibromo-9,9-bis(4-nitrophenyl)-9H-fluorene and 6.8965g (0.05mol) 4-hydroxyphenylboronic acid to a 500ml three-necked flask Add 250ml of tetrahydrofuran (THF), and then add 75ml of 2mol / L potassium carbonate solution, stir magnetically and pass argon, heat the oil bath to 70°C, add 0.02g tetrakistriphenylpalladium, and reflux for 24h. The reaction liquid was poured into water for extraction, a large amount of precipitates precipitated out, filtered through a funnel, and the precipitates were collected, then the filter cake was washed three times with dichloromethane, and a yellow solid was obtained after suction filtration, which was dried in vacuum...
Embodiment 3
[0064] Synthesis of 4,4'-(2,7-bis(4-((E)-1,2-diphenylvinyl)phenyl)-9H-fluorene-9,9-diyl) dianiline:
[0065]
[0066] (1) Synthesize intermediate 2,7-dibromo-9,9-bis(4-nitrophenyl)-9H-fluorene according to Example 1;
[0067] (2) Synthesis of intermediate (E)-l-bromo-4-(1,2-diphenylvinyl)benzene:
[0068] Add 11.6ml (0.1mol) of benzyl chloride and 52.2ml (0.3mol) of triethyl phosphite into a 500ml three-neck flask, magnetically stir and pass argon, heat and reflux in an oil bath at 150°C for 24h, cool to room temperature, and pour Add 300ml of tetrahydrofuran (THF) into the three-neck flask, then add 16.82g of potassium tert-butoxide (t-BuOK 0.15mol) and 20.9g of monobromobenzophenone ((4-bromophenyl)(phenyl)methanone 0.08mol), and magnetically stir for 24h , after the reaction stopped, the reaction solution was poured into water for extraction, and a large amount of white precipitates precipitated out, filtered through a funnel, collected the precipitates, washed 3 times ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com