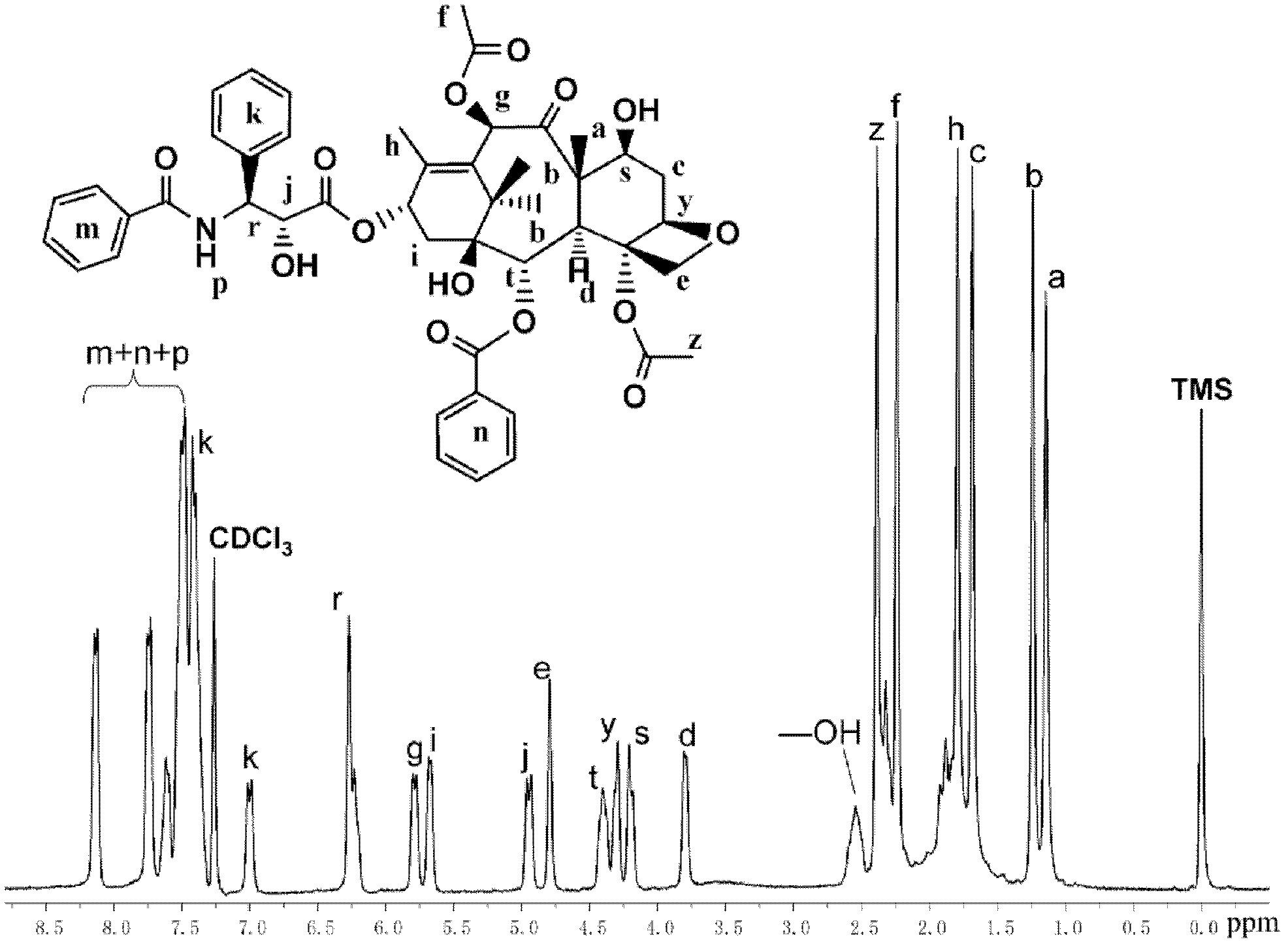
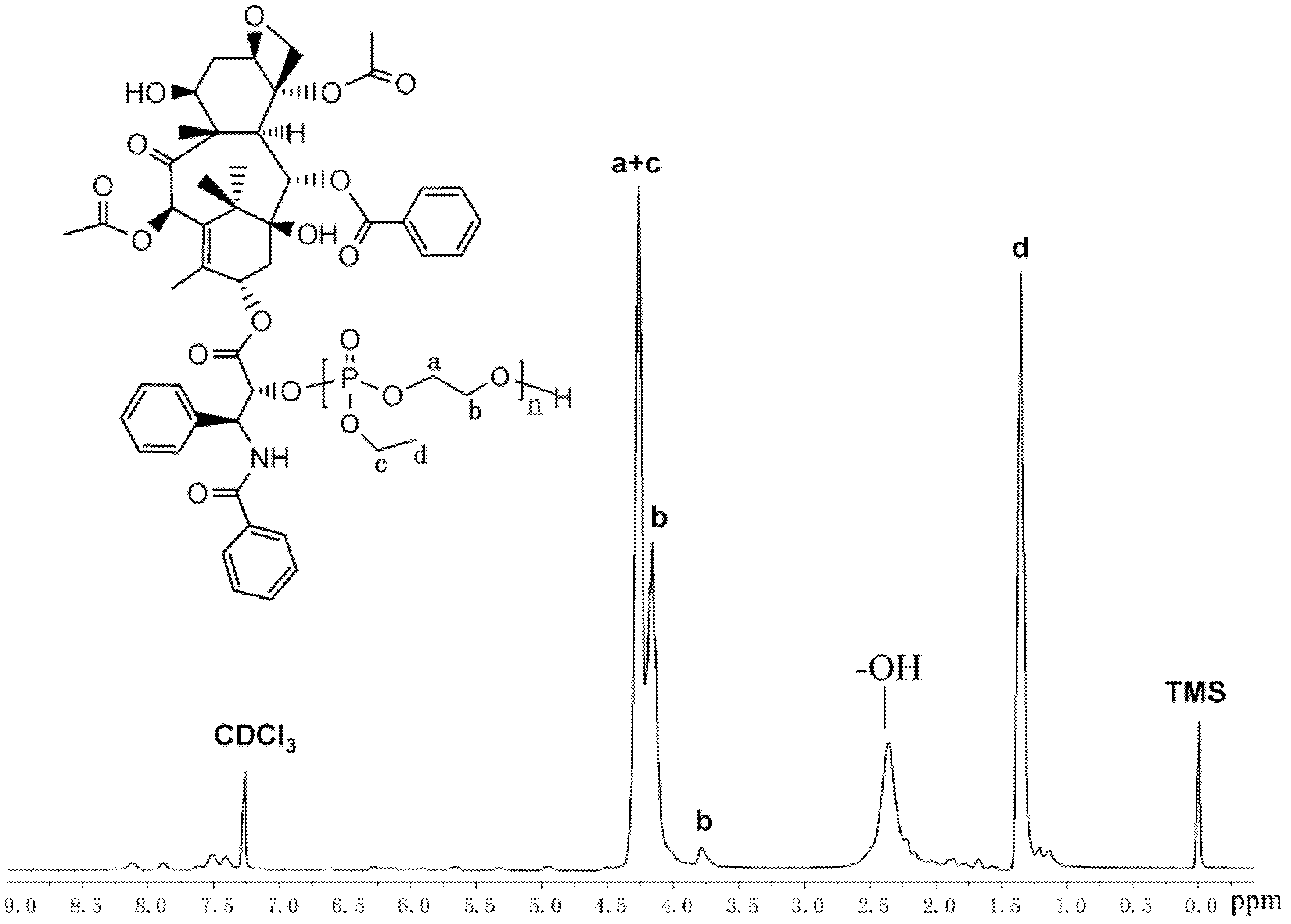
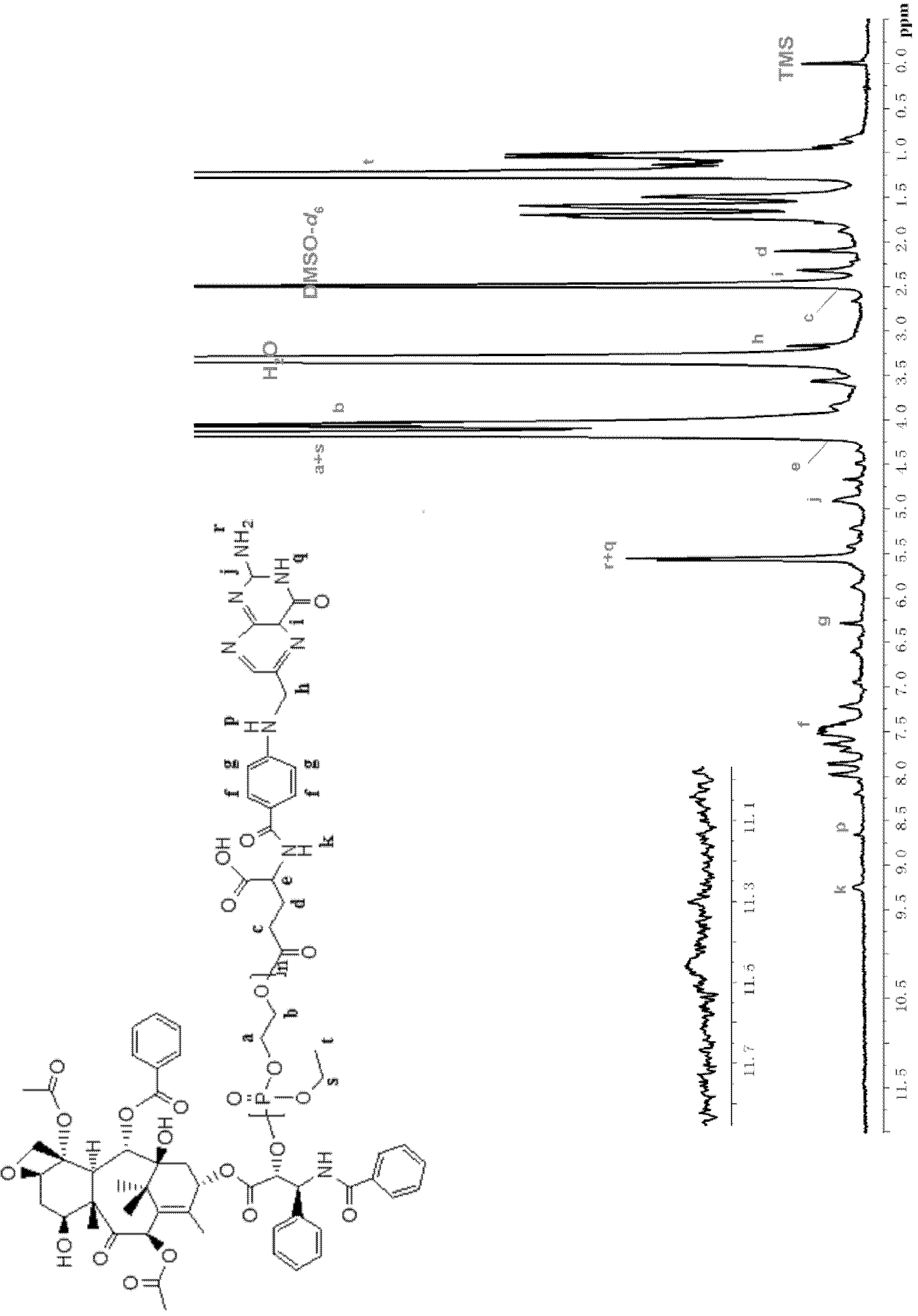
Water-soluble paclitaxel derivative with cell targeting effect and preparation thereof
A paclitaxel derivative and cell-targeting technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve complex preparation processes, changes in drug structure, and affect drug efficacy, etc. problems, to achieve the effect of simple synthesis steps, controlled drug release, and improved water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation method of paclitaxel-bonded polyphosphate drug
[0053] Put the glass syringe, needle, glass stopper, and branch bottle equipped with a stirrer in an oven at 120°C for 12 hours to dry, then take out the syringe and needle and put it in a desiccator to cool, put a stopper on the branch bottle and connect it to the double-row tube Use an oil pump to cool to room temperature, then fill with high-purity argon, and take a breath for three times, and finally fill with argon, and add paclitaxel white powder (0.30g, 0.35 mmol) and stannous octoate (0.07 g, 0.18 mmol) distilled under reduced pressure as a viscous liquid. Exhaust the gas twice, use a dry syringe to extract the solvent o-dichlorobenzene 2mL and tetrahydrofuran 4mL (volume ratio 1:2) into the branch vial, stir and dissolve until the system is clear and transparent. Add phosphate ester monomer 2-ethoxy-2-oxo-1,3,2-dioxaphospholane (EOP) with a molecular weight of 151 g / mol through a syringe, ...
Embodiment 2
[0056] Example 2: Preparation method of paclitaxel-polyphosphate-folate polymer drug (PTX-PEEP-FA)
[0057] Dry the vial with a stirrer, stopper and syringe with a 120°C oven, take it out and put it in a desiccator to cool, add folic acid (0.024g, 0.054mmol) with a molecular weight of 441g / mol to the vial filled with argon, and use Extract 5 mL of distilled and purified dimethyl sulfoxide and 0.5 mL of triethylamine (volume ratio 10:1) into a dry syringe, stir well and dissolve to a transparent yellow solution, add dicyclohexylcarbodiimide (0.014 g, 0.070 mmol ) and N-hydroxysuccinimine (0.008g, 0.070mmol), and the molar ratio of folic acid is 1:1.3, first stirred at room temperature for 12 to 24 hours in the dark. Then the drug macromolecule PTX-PEEP25 (0.20g, 0.043mmol) of polyphosphate-bonded paclitaxel with a number average molecular weight of about 4600g / mol was added to the folic acid reaction system, and the stirring reaction was continued for 24~ 48h.
[0058] After ...
Embodiment 3
[0060] Example 3: Preparation of polymer micelles containing anticancer drugs by dialysis
[0061] Take a 5mg sample of PTX-PEEP in a round bottom flask 25-FA was fully dissolved in 3mL of tetrahydrofuran with stirring, and then 8mL of phosphate buffer solution with a pH value of 7.4 was added dropwise at a rate of 0.5mL / min with a micro-sampling pump under stirring, and then 8 mL of a phosphate buffer solution with a pH value of 7.4 The solution was dialyzed for 24-30 hours to remove tetrahydrofuran, and then the phosphate buffer solution with a pH value of 7.4 was used to dilute to volume in a 25mL volumetric flask, and fully stirred for 48-72 hours. Using a transmission electron microscope to observe the morphology and size of micelles, it can be seen that the polymer drugs bonded by paclitaxel-polyphosphate-folate self-assemble into micelles with a particle size of 80-100nm under simulated physiological conditions. See Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com