Fluorine and vanadium ion-doped lithium iron phosphate material and preparation method thereof
A technology of lithium iron phosphate and vanadium ions, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problem of simultaneously improving the rate performance and discharge potential of lithium iron phosphate, the rate performance and ion conduction of lithium iron phosphate The role of efficiency is not obvious, and the requirements of lithium-ion power batteries cannot be well met, so as to achieve the effect of superior electrochemical performance, cheap raw materials, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
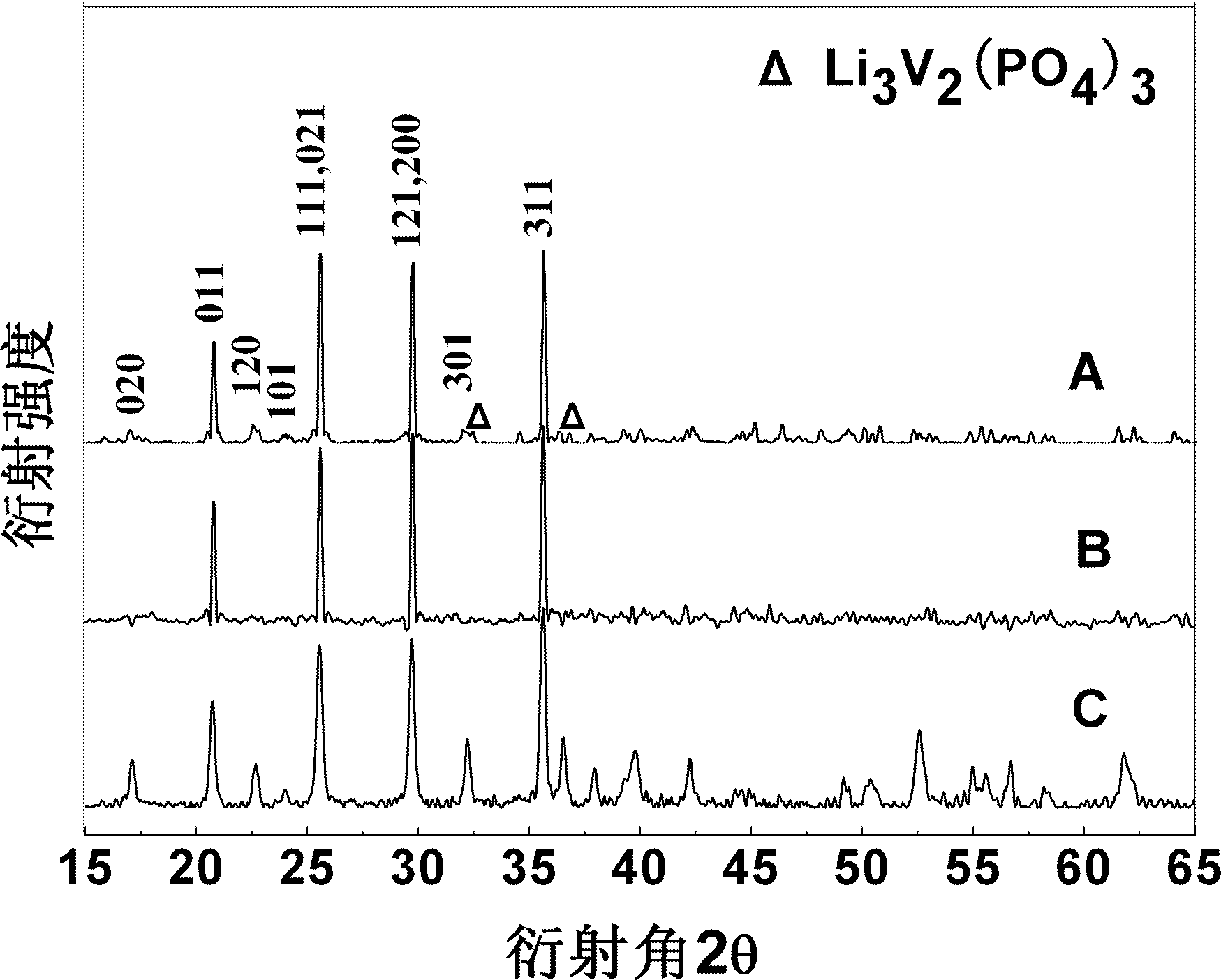
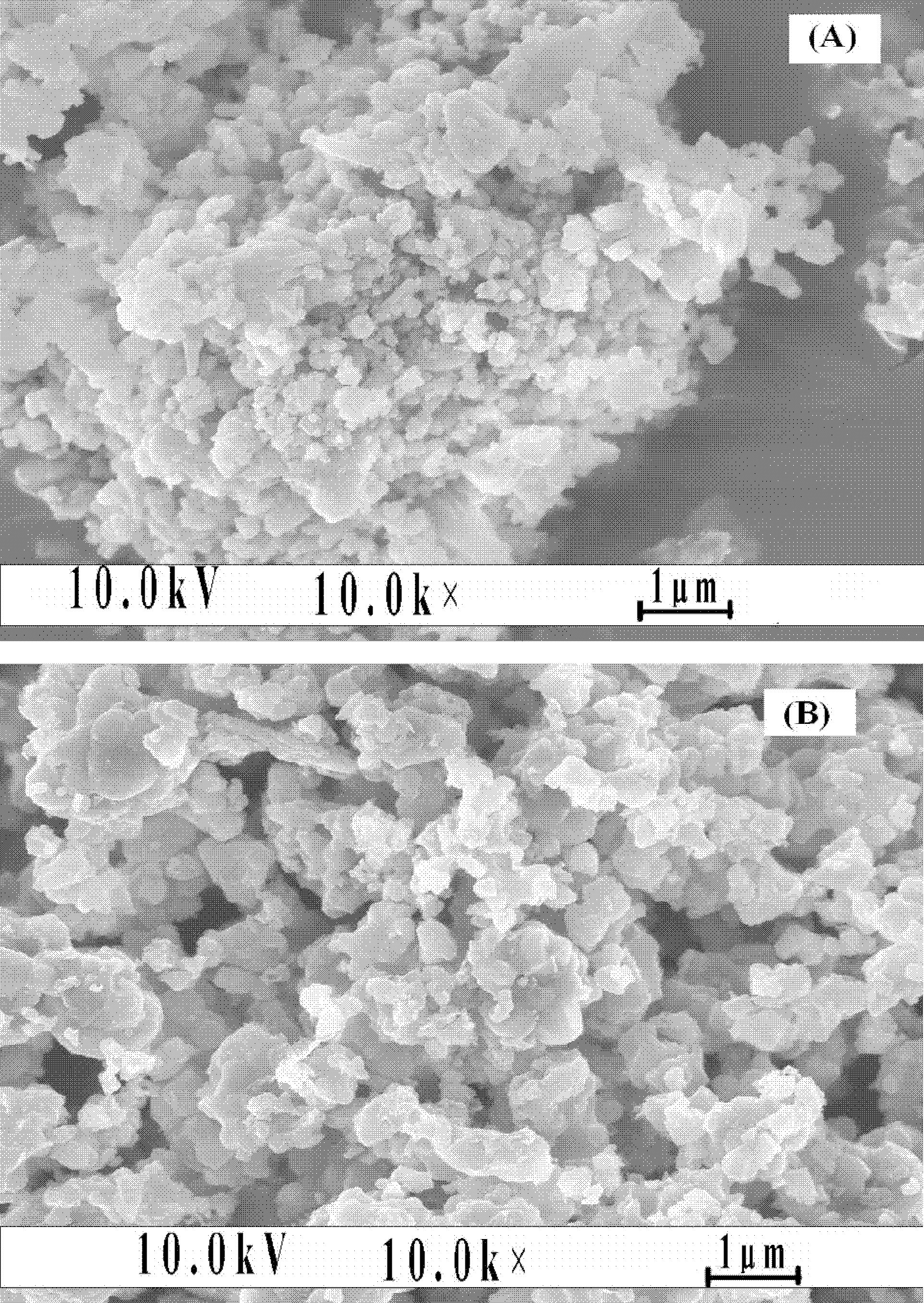
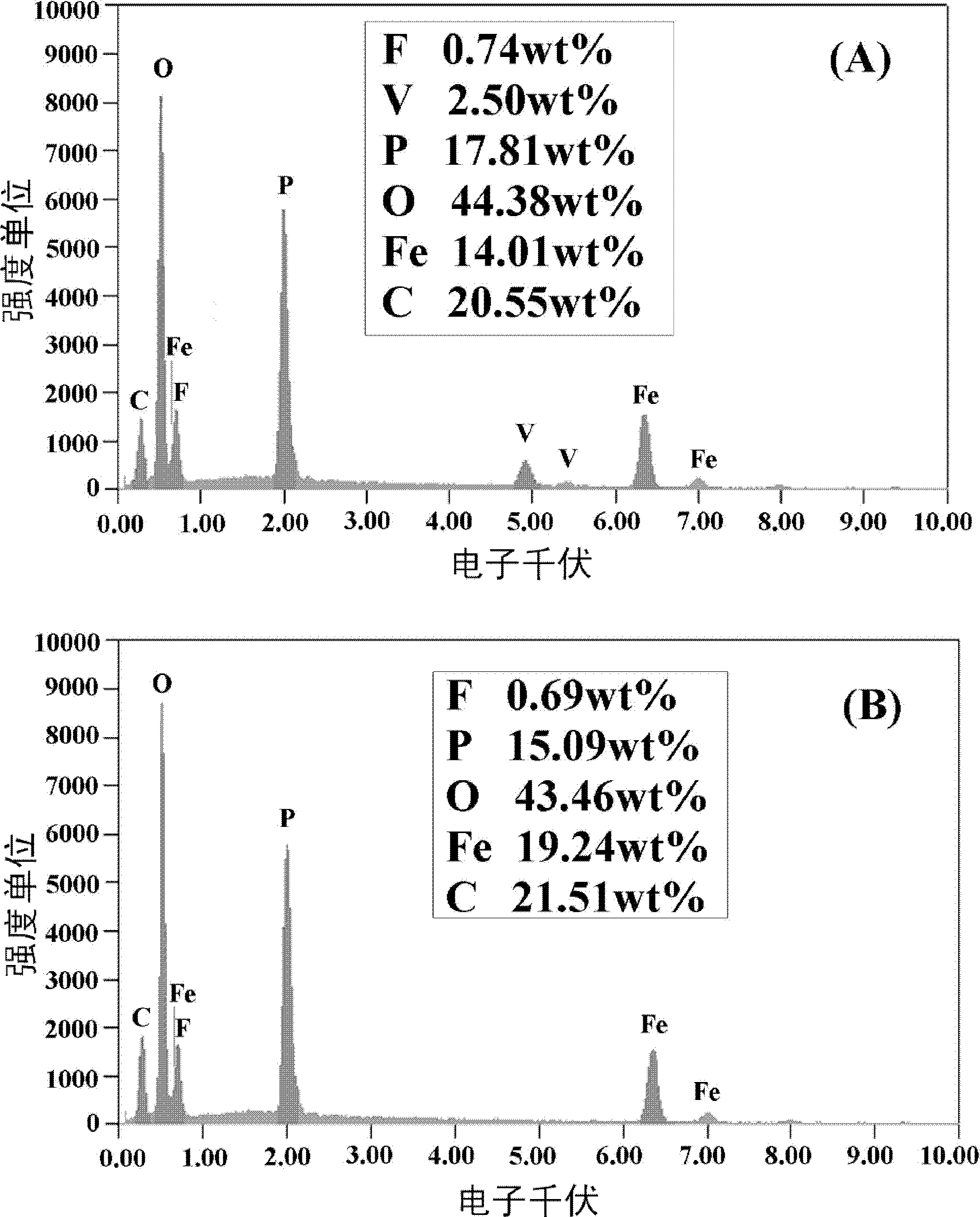
[0036] Combine lithium oxalate, iron phosphate, ammonium dihydrogen phosphate, ammonium vanadate and ammonium fluoride according to the molar ratio of ions Li + : Fe 3+ : V 5+ : PO 4 3- : F - =0.97:0.99:0.01:0.99:0.03 Weigh, add glucose according to 25% of the mass of iron phosphate, then add deionized water according to 300% of the total mass of reactant materials, ball mill and mix for 10 hours to obtain a uniform slurry. Then place it in a pit furnace protected by a nitrogen atmosphere, heat it up to 400°C at a rate of 4°C / min for pre-sintering, and keep it for 2 hours; then heat it up at 10°C / min to 750°C for calcination and keep it for 30 hour. After the furnace is naturally cooled to room temperature, it is taken out and ground and passed through a sieve (400 mesh) to obtain lithium iron phosphate co-doped with fluorine and vanadium ions.
[0037] The fluorine and vanadium ion co-doped lithium iron phosphate material prepared by the above method is used as the positive elec...
Embodiment 2
[0039] Combine lithium carbonate, ferric oxide, diammonium hydrogen phosphate, vanadium pentoxide and ammonium fluoride in the molar ratio of Li + : Fe 3+ : V 5+ : PO 4 3- : F - =1:0.9:0.1:0.95:0.15 Weigh, add soluble starch according to 5% of the mass of ferric oxide, then add absolute ethanol according to 150% of the total mass of reactant materials, ball mill and mix for 6 hours to obtain a uniformly mixed The slurry is then placed in a pit furnace protected by a nitrogen atmosphere. The temperature is raised to 350°C at a heating rate of 2°C / min for pre-sintering, and the temperature is kept for 10 hours; then it is heated at 5°C / min to 650°C for calcination , Keep warm for 24 hours. After the furnace is naturally cooled to room temperature, it is taken out and ground and passed through a sieve (400 mesh) to obtain a lithium iron phosphate co-doped with fluorine and vanadium ions. For comparison purposes, fluorine-doped lithium iron phosphate materials and lithium iron phos...
Embodiment 3
[0043] Lithium acetate, iron nitrate, ammonium phosphate, ammonium vanadate and lithium fluoride according to the molar ratio of ions Li + : Fe 3+ : V 5+ : PO 4 3- : F - =1.02:0.95:0.05:0.9:0.3 Weigh, add sucrose according to 50% of the mass of ferric nitrate, then add acetone according to 100% of the total mass of reactant materials, ball mill and mix for 8 hours to obtain a uniform slurry, and then It is placed in a pit furnace protected by a nitrogen atmosphere, heated to 300°C at a heating rate of 1°C / min for pre-sintering and kept for 6 hours; then heated at 15°C / min to 550°C for calcination and kept for 18 hours. After the furnace is naturally cooled to room temperature, it is taken out and ground and passed through a sieve (400 mesh) to obtain lithium iron phosphate co-doped with fluorine and vanadium ions.
[0044] The fluorine and vanadium ion co-doped lithium iron phosphate material prepared by the above method is used as the positive electrode active material to make th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com