Method for separating and purifying blood coagulation factor VIII
A coagulation factor, separation and purification technology, applied in the field of separation and purification of coagulation factor VIII, can solve problems such as affecting the activity of the final product, easy dissociation, and short half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
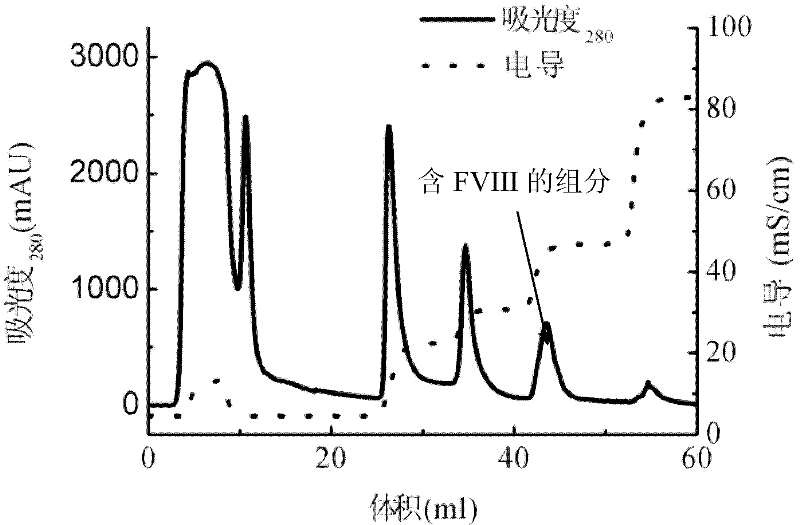
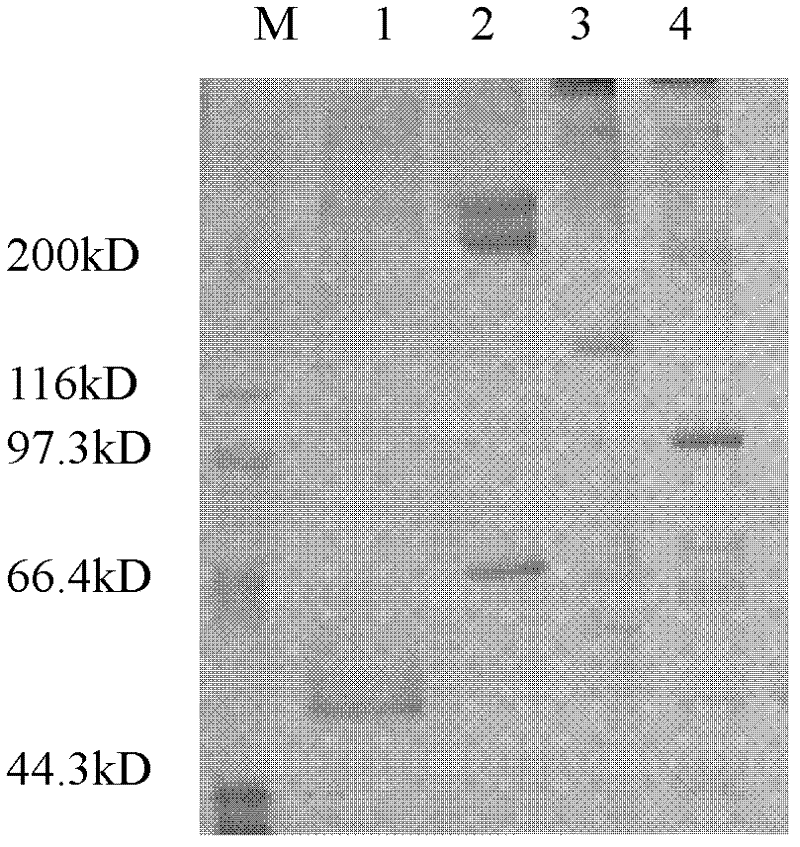
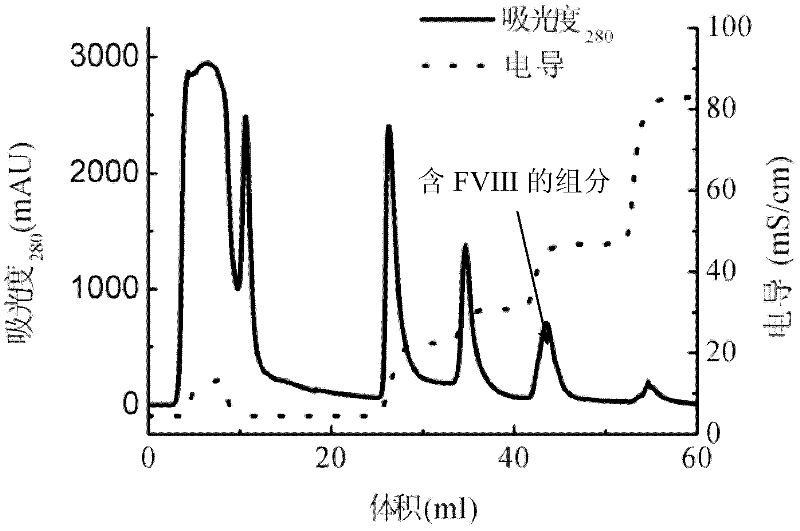
[0031] Take frozen healthy human plasma and melt it in a water bath at 37°C, and shake it continuously to fully dissolve FVIII, etc. After completely dissolving, inject 40mL into a 20mM, pH 7.5 citrate buffer containing 120mM glycine and 1mM calcium chloride. POROS 50HQ ion-exchange chromatography column, column inner diameter 2.6 cm, column bed height 10 cm, chromatography equipment GE AKTA Explorer 100, ultraviolet 280nm wavelength absorption detection, dimensionless flow rate 0.02S -1 After the sample injection, continue to wash with the above citrate buffer until the 280nm UV absorption signal returns to the baseline, and then use 20mM, pH 7.5 citrate buffer containing 120mM glycine, 1mM calcium chloride and 1M sodium chloride for gradient Elution, the concentration gradients are 20%, 30%, 50% and 100%, respectively, two column volumes are eluted for each gradient, and 35mL of the elution peak of the 50% gradient is collected, the protein concentration is 0.73g / L, and the s...
Embodiment 2
[0033] Component I obtained from Cohn precipitation of healthy human plasma was melted in a 37°C water bath, and continuously oscillated to fully dissolve FVIII, etc. After complete dissolution, inject 20mL of citrate containing 120mM glycine and 1mM calcium chloride at pH 7.5 to use 20mM Diethylamine-poly(glycidyl methacrylate-divinylbenzene-triallyl trimeric isocyanurate) type anion exchange chromatography column with well-balanced buffer solution, the inner diameter of the column is 2.6 cm, and the height of the column bed is 5 cm, chromatography equipment GE AKTA Explorer 100, ultraviolet 280nm wavelength absorption detection, dimensionless flow rate 0.01S -1 After loading the sample, continue to wash with the above citrate buffer until the 280nm UV absorption signal returns to the baseline, and then use 20mM, pH 7.5 citrate buffer containing 120mM glycine, 1mM calcium chloride and 1M sodium chloride for gradient Elution, the concentration gradients were 25%, 60% and 100%,...
Embodiment 3
[0035] Take 3g of healthy human plasma cryoprecipitate and dissolve it in 50ml of 20mM, pH7.5 citrate buffer containing 120mM glycine and 1mM calcium chloride, and take 40mL of the dissolved solution and inject it into the POROS50Q ion exchange that has been balanced with the above citrate buffer. In the chromatographic column, the inner diameter of the column is 2.6 cm, the height of the column bed is 10 cm, the chromatographic equipment is GE AKTA Explorer 100, the ultraviolet 280nm wavelength absorption detection, the dimensionless flow rate 0.005S -1 After loading the sample, continue to wash with the above citrate buffer until the 280nm UV absorption signal returns to the baseline, and then use 20mM, pH 7.5 citrate buffer containing 120mM glycine, 1mM calcium chloride and 1M sodium chloride for gradient Elution, the concentration gradients were 35%, 55% and 100%, respectively, each gradient eluted two column volumes, and collected 33mL of 50% gradient elution peak, the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com