Preparation method of 2-hydroxy-2-arylthioacetamide
A technology of arylthioacetamide and hydroxythioamide, which is applied in the field of organic compound synthesis, can solve the problems of complicated operation, harsh conditions, high price and the like, and achieves the effects of simple experimental operation, mild reaction conditions and convenient handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
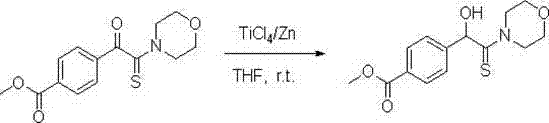
[0028] 1. Add zinc powder (0.78 g, 12 mmol) and 20 mL of anhydrous THF to a 100 mL three-necked flask, under nitrogen protection, stir at room temperature, and slowly inject TiCl 4 (0.7 mL, 6 mmol), the mixture was refluxed for 2 h, and cooled to room temperature to obtain a low-valent titanium reagent in black paste, slowly adding a solution of 2-carbonylthioamide (2 mmol) dissolved in 10 mL THF, and the addition was completed. React at room temperature for 5-10 min, and TLC detects that the reaction is complete.
[0029] 2. After the reaction, decompose with 100 mL of 3% hydrochloric acid, and then extract with chloroform (3×50 mL). The organic layers were combined, washed with water until neutral (2×50 mL), dried over anhydrous sodium sulfate, filtered to remove the desiccant, and evaporated to remove the solvent under reduced pressure to obtain a crude product, which was recrystallized from ethanol with a yield of 91%. The reaction equation is expressed as follows:
[00...
Embodiment 2
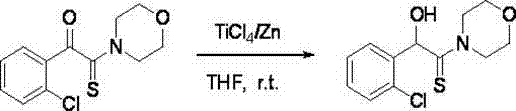
[0032] 1. Add zinc powder (0.78 g, 12 mmol) and 20 mL of anhydrous THF to a 100 mL three-necked flask, under nitrogen protection, stir at room temperature, and slowly inject TiCl 4 (0.7 mL, 6 mmol), the mixture was refluxed for 2 h, cooled to room temperature to obtain low-valent titanium reagent in black paste, slowly added 2-carbonylthioamide (2 mmol) dissolved in 10 mL THF solution, added complete. React at room temperature for 5-10 min, and TLC detects that the reaction is complete.
[0033] 2. After the reaction, decompose with 100 mL of 3% hydrochloric acid, and then extract with chloroform (3×50 mL). The organic layers were combined, washed with water until neutral (2×50 mL), dried over anhydrous sodium sulfate, filtered to remove the desiccant, and evaporated to remove the solvent under reduced pressure to obtain a crude product, which was recrystallized from ethanol with a yield of 89%. The reaction equation is expressed as follows:
[0034]
Embodiment 3
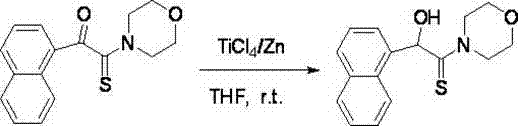
[0036] 1. Add zinc powder (0.78 g, 12 mmol) and 20 mL of anhydrous THF to a 100 mL three-necked flask, under nitrogen protection, stir at room temperature, and slowly inject TiCl 4 (0.7 mL, 6 mmol), the mixture was refluxed for 2 h, cooled to room temperature to obtain low-valent titanium reagent in black paste, slowly added 2-carbonylthioamide (2 mmol) dissolved in 10 mL THF solution, added complete. React at room temperature for 5-10 minutes, and TLC detects that the reaction is complete.
[0037] 2. After the reaction, decompose with 100 mL of 3% hydrochloric acid, and then extract with chloroform (3×50 mL). The organic layers were combined, washed with water until neutral (2×50 mL), dried with anhydrous sodium sulfate, filtered to remove the desiccant, and evaporated to remove the solvent under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography [eluent V (acetone): V (petroleum ether) = 1:3] to isolate the product with a y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com