Transformation of acidic beta-mannase gene and construction of engineering bacteria of acidic beta-mannase gene
A mannanase and acid technology, applied in the field of genetic engineering, can solve the problems of low yield, complicated fermentation process and high cost, and achieve the effect of good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Obtaining the whole gene of β-mannanase from Aspergillus niger CBS 513.88
[0046] Extraction of gene fragments
[0047]The β-mannanase gene An15g07760 of Aspergillus niger CBS 513.88 strain was obtained from the NCBI database, its sequence was analyzed and the gene sequence was optimized according to the codon preference and mRNA structure of Pichia pastoris, and the SignalP 3.0 Server online analysis software was used to analyze the gene sequence. The signal peptide of the protein encoded by the gene and the gene sequence encoding the signal peptide are excised, and the XhoI restriction site (C^TCGAG) and CG protection base are added to the 5' end, and the XbaI (T^CTAGA) enzyme digestion is added to the 3' end Site and protection base GC to obtain optimized β-mannanase gene. Design three primers:
[0048] Fn-Man: GACCGCTCGAGAAGAGAGCTTCTAATC;
[0049] Rn-Man1: GCTCTAGAGCAGCACCTTCCCAATTC;
[0050] Rn-Man2: GCTCTAGAGCTTAAGCACCTTCCCAATTC.
[0051] Synthes...
Embodiment 2
[0074] Example 2 Construction of engineering bacteria expressing Aspergillus niger β-mannanase
[0075] Construction of β-mannanase Gene Amplification Vector and Gene Amplification
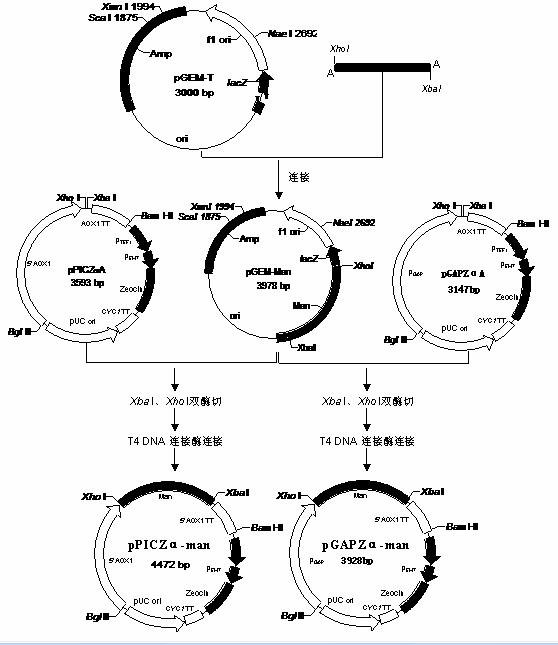
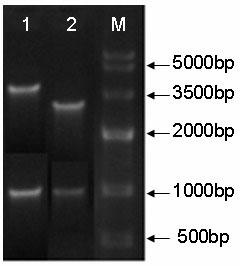
[0076] The complete β-mannanase gene obtained by Assembly PCR and LCR was connected to the pGEM-T vector, and then transferred into DH5α competent cells, the method was the same as above. After the white spots were picked and identified as positive clones by colony PCR, they were sent to Shanghai Sunny Biotechnology Co., Ltd. for sequencing. The β-mannanase gene recombinant plasmid clone without mutation identified by sequencing was preserved for future use.
[0077] Construction of expression vector of β-mannanase gene Pichia pastoris
[0078] Methanol-inducible expression vector pPICZα-man
[0079] The DH5α clone of the recombinant β-mannanase gene was cultivated, and pGEM-man was extracted by alkaline lysis (using the specific method described in the book "Molecular Cloning Experiment Guid...
Embodiment 3
[0098] Example 3 Inducible expression of Aspergillus niger β-mannanase
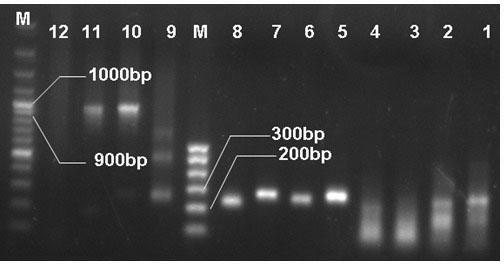
[0099] Pick a stable recombinant monoclonal strain, inoculate it into a 250mL shake flask containing 25mL BMGY, and cultivate it at 28-30°C and 250-300rpm until OD600=2-6 (about 16-18 hours). Inoculate 25mL medium into a 3L shake flask containing 1L BMGY, shake vigorously at 28-30°C (250-300rpm), and reach the logarithmic growth phase (OD600=2-6). Use a sterilized centrifuge tube and centrifuge at 1500-3000g at room temperature for 5min to collect the cells. Remove the supernatant and resuspend the cells with BMMY to OD600=1.0 (2-6). Divide the medium into three 3L septum shaker flasks, cover with 2 layers of sterile gauze or cheese cloth, and put them in a shaker at 28-30°C to continue culturing. Every 24 hours, add methanol to a concentration of 0.5% until the optimal induction time is reached. Bacteria liquid samples were taken according to time points ((24 h, 48 h, 60 h, 72 h, 96 h), the sampling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com