Photodynamic treatment medicament, medical composition and preparation method thereof
A technology of drugs and compounds, which is applied in the field of photodynamic therapy drugs, pharmaceutical compositions and their preparation, can solve the problems of limiting the therapeutic effect of photodynamic therapy and the inability of external light sources to penetrate deep tissues, and achieve the effect of avoiding damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
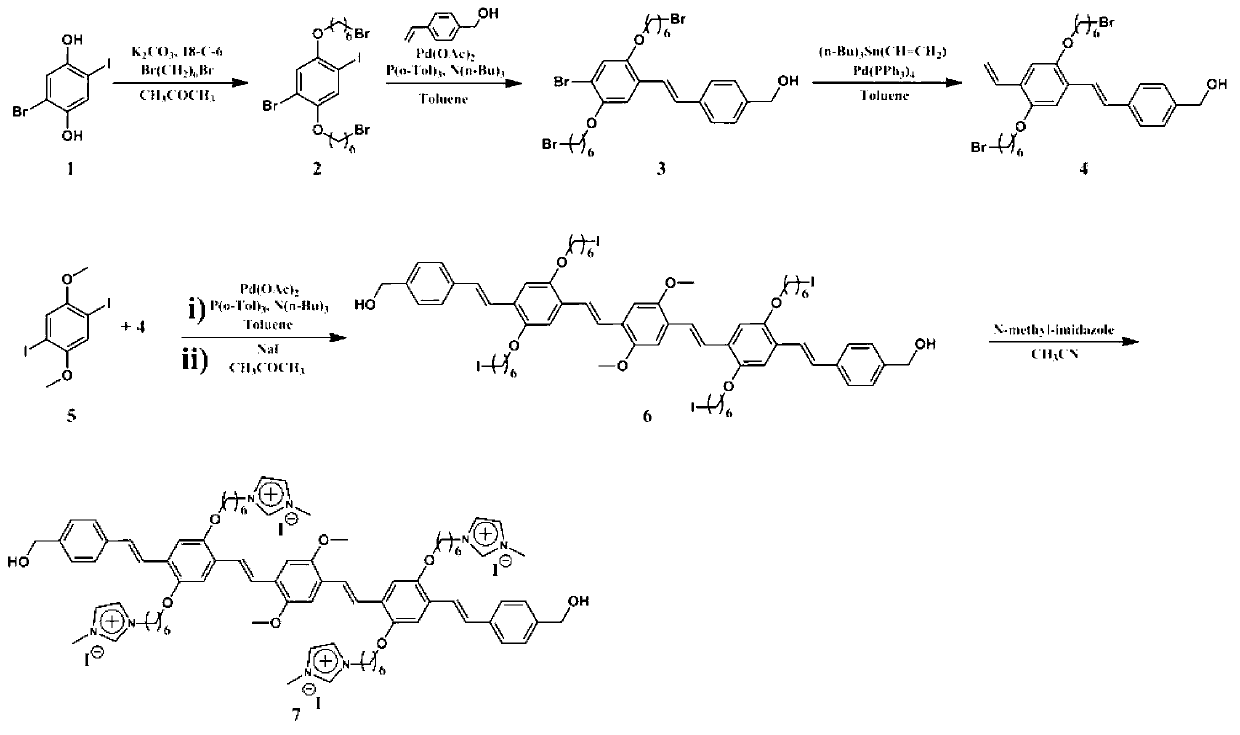
[0053] The synthesis of compound OPV shown in embodiment 1, formula I
[0054] For the synthetic route map, see figure 1 .
[0055]
[0056] 1. Synthesis of Compound 2 (Formula 2)
[0057] Add 1.7g of compound 1 (Formula 1), 70mL of acetone deoxygenated, 5.6g of potassium carbonate, 0.14g of 18-crown-6 and 20g of 1,6-dibromohexane into a 250mL single-necked bottle, and heat up to 75°C for 3 days . Add 100mL of water to quench the reaction, then add 100mL of dichloromethane to extract the product, the organic phase is dried with anhydrous magnesium sulfate, and the solvent is evaporated and then separated on a silica gel column (eluent: dichloromethane:petroleum ether=1:5, v / v) Afterwards, 2.5 g of white solid were obtained. Characterization of the product: 1H NMR (400MHz, CDCl3) δ7.27 (s, 1H), 6.98 (s, 1H), 3.95 (m, 4H), 3.43 (m, 4H), 1.91 (m, 4H), 1.82 ( m, 4H), 1.54 (m, 8H).13C NMR (100MHz, CDCl3) δ152.62, 150.50, 124.37, 117.19, 112.66, 33.91, 32.78, 29.82, 29.07, ...
Embodiment 2
[0070] Example 2, the pharmaceutical composition composed of luminol luminescent system and OPV is used for killing cancer cells
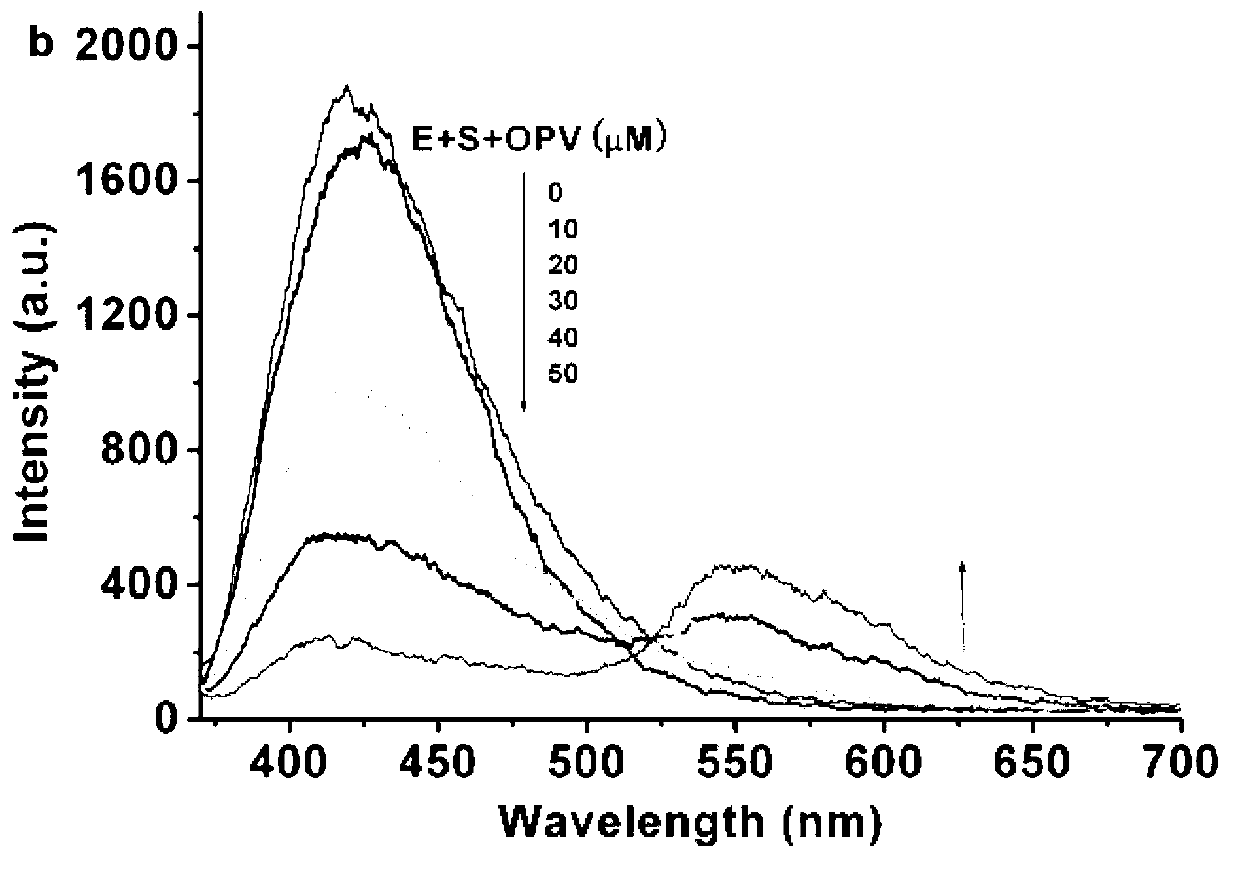
[0071] (1) Determination of bioluminescence energy transfer spectrum between luminol and OPV
[0072] Add 3 μL 1 mg / mL HRP (horseradish peroxidase) pH7.0 sodium dihydrogen phosphate solution, 10 μL 20 mM luminol, 10 μL 50 mM p-iodophenol aqueous solution and different concentrations of OPV to 967 μL pH9.0 sodium carbonate buffer (to make the final concentrations of 10 μM, 20 μM, 30 μM, 40 μM and 50 μM respectively), after vortexing for 5 s, add 10 μL of 50 mM hydrogen peroxide aqueous solution and vortex for 2 s to measure the luminescence spectrum immediately, and the spectral range is 370 nm to 700 nm. See figure 2 .
[0073] (2), culture of HeLa cells
[0074] HeLa cells were cultured in DMEM medium containing 10% bovine serum in a constant temperature incubator containing 5% carbon dioxide at 37°C.
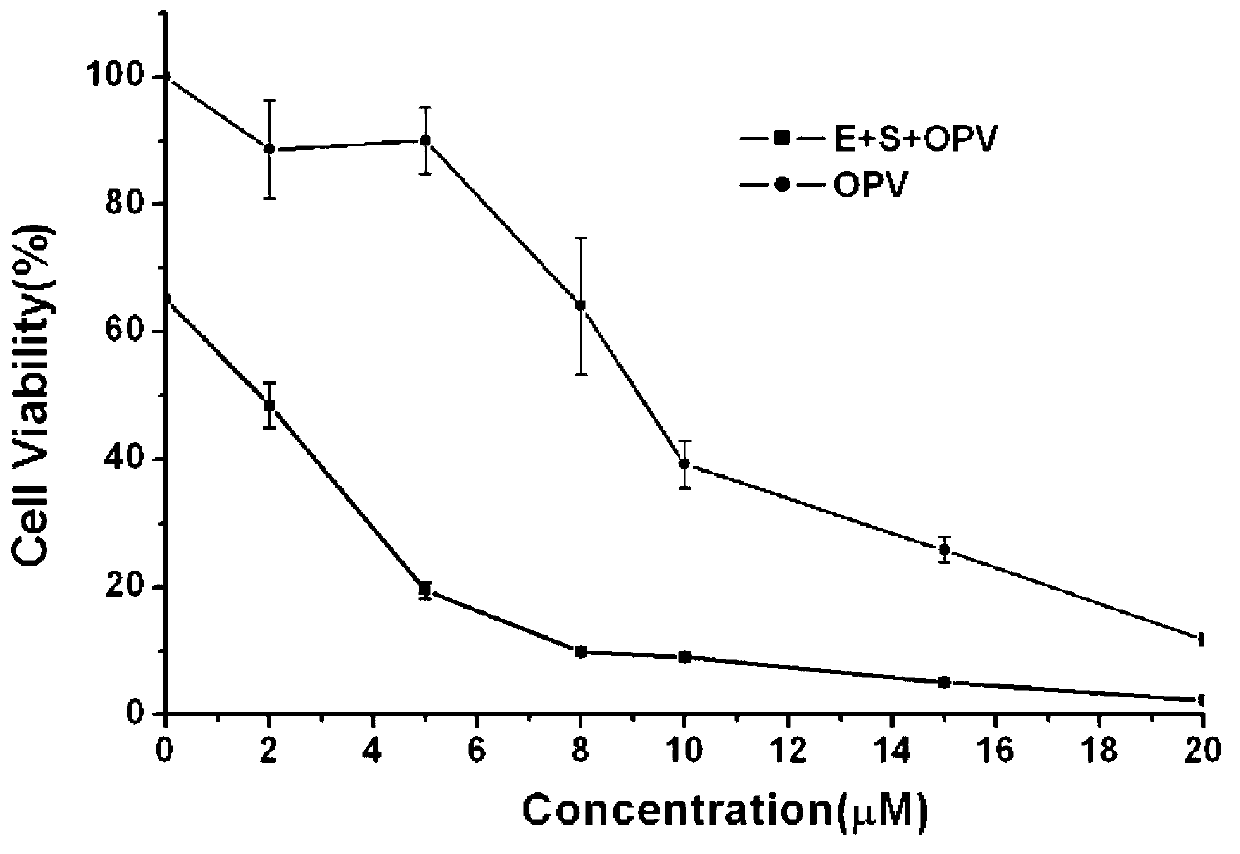
[0075] (3), HeLa cell survival analysis...
Embodiment 3
[0079] Example 3. The composite drug composed of luminol luminescent system and OPV is used for tumor suppression in mice
[0080] (1) Formation of HeLa cell tumor model in nude mice
[0081] Will contain 2 x 10 6 200 μL PBS buffer solution of HeLa cells was subcutaneously injected in the axilla of the left forelimb of 14-15 g female nude mice, and 40 mice were inoculated.
[0082] (2), administration in groups
[0083] According to the sequence of injected cells, they were divided into 4 groups, with 10 rats in each group, that is, each group had the first injection and the second injection. The first group is a blank control group, only injected with 100 μL of normal saline; the second group is a positive control group, injected with enzymes and substrates, first injected with 50 μL of HRP (0.01 mg / mL), luminol luminescence enhancer (p-iodophenol , 1.25mM) and luminol (0.5mM) normal saline solution, then injected 50μL hydrogen peroxide normal saline solution (2mM); the th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com