Rhodamine-B hydrazide derivative containing 1,2,4-triazole structural unit, preparation method and application thereof
A technology of hydrazide derivatives and structural units, applied in the field of rhodamine B hydrazide derivatives and its preparation, to achieve good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Rhodamine B Thiobishydrazide Derivatives
[0025]Add 4.50g (9.85mmol) rhodamine B hydrazide to 130mL acetonitrile solution containing 2.09g (10.81mmol) p-methoxybenzoyl isothiocyanate, heat the mixture to reflux for 20 minutes, then cool down to room temperature , a large amount of white solids were precipitated, filtered under reduced pressure, the filter cake was washed with cold acetonitrile, and dried to obtain 6.26 g of rhodamine B thiobishydrazide derivative 3 as a white or light pink solid, yield: 97.7%.
[0026] Infrared spectrum measurement: IR(KBr), υ: 3282,3081,2970,2931,2893,2870,1711,1662,1633,1612,1510,1425,1375,1356,1330,1261,1221,1186,1118,1079 ,1023,857,816,785,763,698,635,606,578cm -1 .
[0027] H NMR spectrum determination: 1 H NMR (CDCl 3 ,400MHz),δ(ppm):1.16(t,12H,NCH 2 CH 3 ,J=7.0Hz),3.32(q,8H,NCH 2 CH 3 ,J=7.1Hz),3.84(s,3H,OCH 3 ),6.33(dd,2H,Xanthene–H,J=8.9,2.1Hz),6.36(d,2H,Xanthene–H,J=2.0Hz),6.78(d,2H,Xanthene–...
Embodiment 2
[0030] Example 2 Preparation of rhodamine B hydrazide derivatives containing 1,2,4-triazole structural units
[0031] 1.22g (1.88mmol) of rhodamine B thiobishydrazide derivative 3 was dissolved in 50mL of chloroform, and then 550mg (9.34mmol) of 85wt% hydrazine hydrate was added to the above solution. The reaction mixture was heated to reflux, and the reaction was continued for 1 hour. After the reaction was complete, the system was cooled down, diluted with 50 mL of chloroform, washed with water, left to stand, separated into layers, separated, the organic phase was dried with anhydrous magnesium sulfate, filtered with suction, and the filtrate was concentrated to obtain the crude product .
[0032] Purification by silica gel column chromatography (1:2 petroleum ether-ethyl acetate) yielded 314 mg of light purple compound 4, which is a rhodamine B hydrazide derivative containing a 1,2,4-triazole structural unit.
[0033] Infrared spectrum measurement: IR(KBr), υ: 3209,3077,2...
Embodiment 3
[0039] Embodiment 3 UV-Vis Spectrophotometry and Fluorescence Spectrophotometry Test
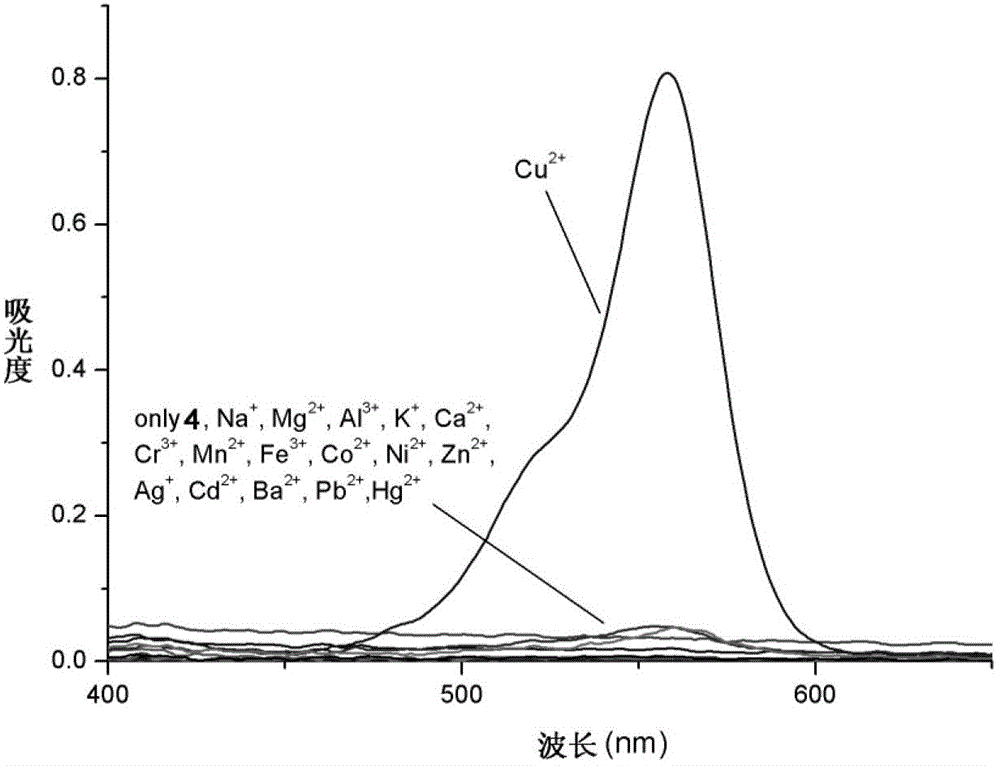
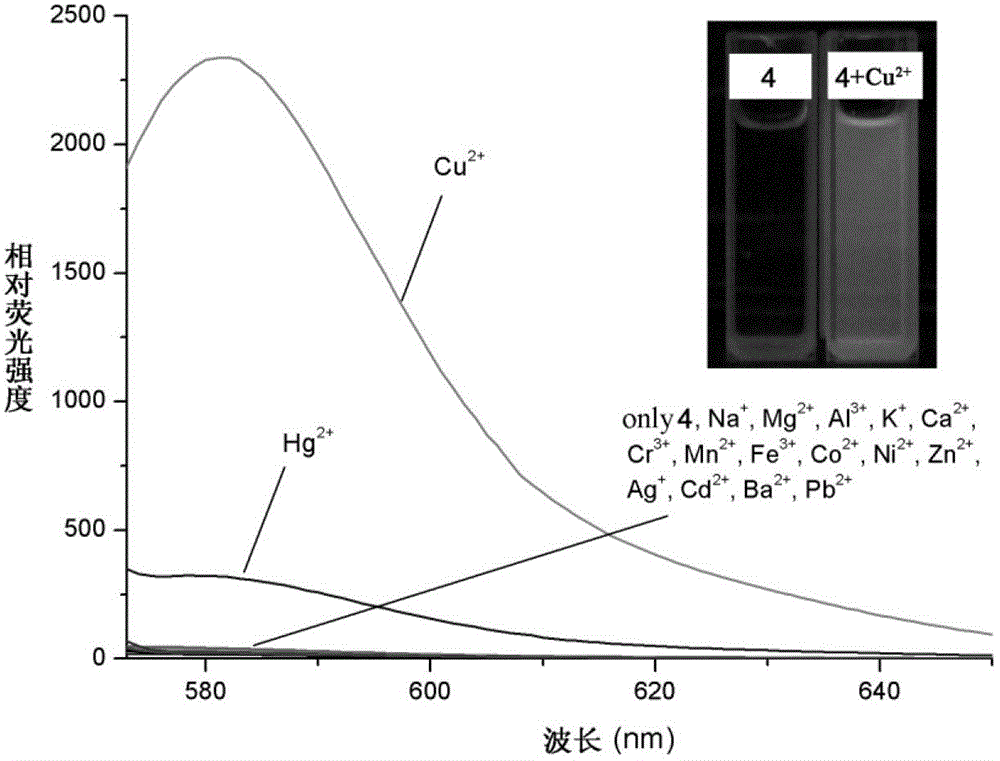
[0040] Use a micro-syringe to add 10 mL of the prepared rhodamine B hydrazide 1,2,4-triazole derivative (4) to ethanol / HEPES buffer solution (20 mM HEPES, pH=7.2, 1:1, volume ratio ) solution by adding 10 equivalents of Na + ,Mg 2+ ,Al 3+ ,K + , Ca 2+ ,Cr 3+ ,Mn 2+ , Fe 3+ ,Co 2+ , Ni 2+ ,Zn 2+ , Ag + ,Cd 2+ , Ba 2+ ,Pb 2+ ,Hg 2+ and Cu 2+ The ionic water solution was tested by UV-Vis spectrophotometry and fluorescence spectrophotometry respectively.
[0041] The result is as image 3 , showing the effect of the 1,2,4-triazole derivative (4) of rhodamine B hydrazide on Cu 2+ It has very good selectivity, and the contrast before and after adding copper ions shows that the fluorescence is enhanced by 120 times, and has a strong fluorescence enhancement effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com