Preparation method of intermediate for synthesis of micafungin and derivative thereof
A compound and ketone compound technology, applied in the field of drug synthesis, can solve the problems of expensive metal reagents, short reaction routes, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of compound b
[0046] Compound b synthesis consists of the following three steps:
[0047] (1) Synthesis of compound 1
[0048]
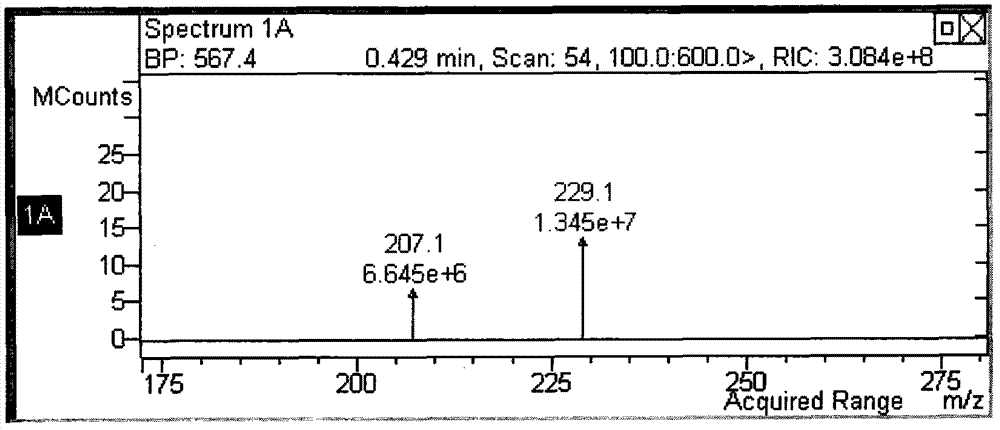
[0049] Dissolve 23g (136g / mol, 0.17mol) of p-hydroxyacetophenone, 28g (151g / mol, 0.19mol) of bromopentane, and 28g (138g / mol, 0.21mol) of anhydrous potassium carbonate in 115mL of dimethylformaldehyde Stir in amide (DMF) at an internal temperature of 65-70°C for 4 hours. The reaction solution was a white suspension. After the reaction was completed, it was cooled to room temperature, and 210 mL of petroleum ether and 210 mL of water were added in sequence, separated by shaking, and the petroleum ether layer was retained. Wash the petroleum ether layer successively with 120ml of 0.5mol / L sodium hydroxide solution and 120ml of saturated brine, add anhydrous magnesium sulfate to dry the petroleum ether for 30min, remove the magnesium sulfate by filtration and evaporate the petroleum ether to obtain a colorless oil...
Embodiment 2
[0059] Embodiment 2: the synthesis of compound a
[0060]
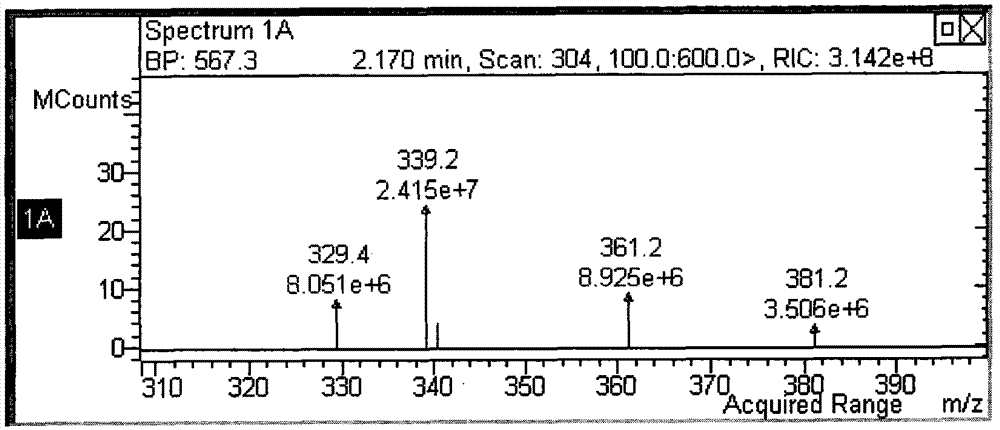
[0061] Compound 3 crude product 1.05g (351g / mol, 0.003mol), HOBT 0.51g (135g / mol, 0.0036mol), EDCI 1.05g (192g / mol, 0.0045mol) were dissolved in 30ml of dichloromethane, stirred at room temperature for 5 hours, The reaction starting materials gradually dissolved. After the reaction was completed, 30 ml of water was added to wash the reaction liquid, the liquid was separated, the organic phase was retained, anhydrous magnesium sulfate was added to dry the organic phase, and the organic phase was separated through a silica gel column by filtration. The eluent was dichloromethane:n-hexane:ethyl acetate=10:10:1~5:5:1 gradient elution, and 0.72g of compound a was obtained.
[0062] 1 H-NMR (400MHz, CDCl 3 )0.95(3H, t, J=6.7Hz), 1.36-1.51(4H, m), 1.80-1.87(2H, m), 4.03(2H, t, J=6.4Hz), 6.81(1H, s), 7.01 (2H, d, J = 8.4Hz), 7.45-7.60 (3H, m), 7.79 (2H, d, J = 8.3Hz), 8.11 (2H, d, J = 8.0Hz), 8.13 (1H, s ), 8.39 (2H, ...
Embodiment 3
[0063] Embodiment 3: N-hydroxy p-toluenesulfonamide (TsNHOH) is synthesized
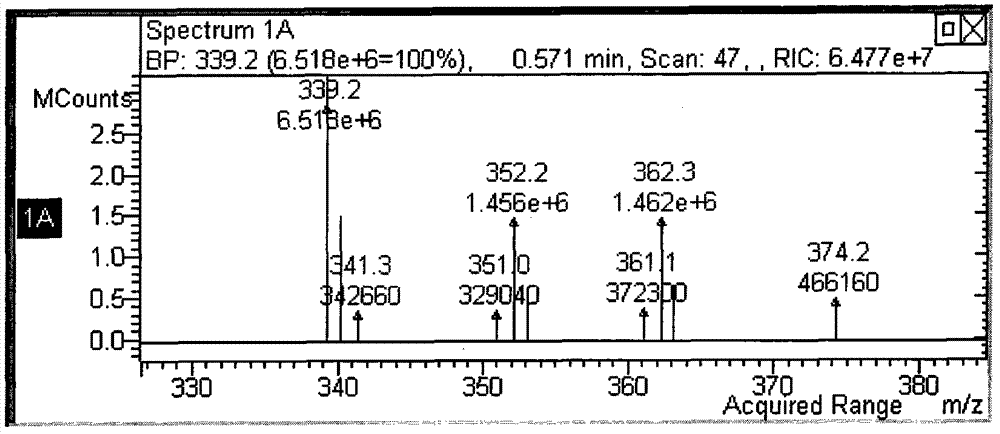
[0064] Mixed solution A was prepared by dissolving 60 g (190 g / mol, 0.3 mol) of p-toluenesulfonyl chloride and 200 ml (101 g / mol, 1.5 mol) of triethylamine in 200 ml of deionized water. Dissolve 62 g of hydroxylamine hydrochloride (69 g / mol, 0.9 mol) in 200 ml of THF, cool in an ice bath and control the temperature at 0-5°C, and add it dropwise into the mixture A with vigorous stirring. Rising to room temperature and stirring for 2 hours, the reaction was completed, the liquid was separated, and the upper organic phase was collected. 100ml of 10% dilute hydrochloric acid was added to the organic phase, and the mixed solution was evaporated to dryness by rotary evaporation, and a large amount of white solid was precipitated. After filtration, 23g of the white solid product hydroxy-p-toluenesulfonamide was obtained, with a yield of 39%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com