Medical porous metal material for replacing weight-bearing bone tissues and preparation method of medical porous metal material
A porous metal and bone tissue technology, applied in tissue regeneration, medical science, prosthesis, etc., can solve the problems of insufficient purity of finished products, carbon skeleton residues, and reduced biological safety, and achieve high qualified rate of finished products and uniform distribution of pores , Improve the effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
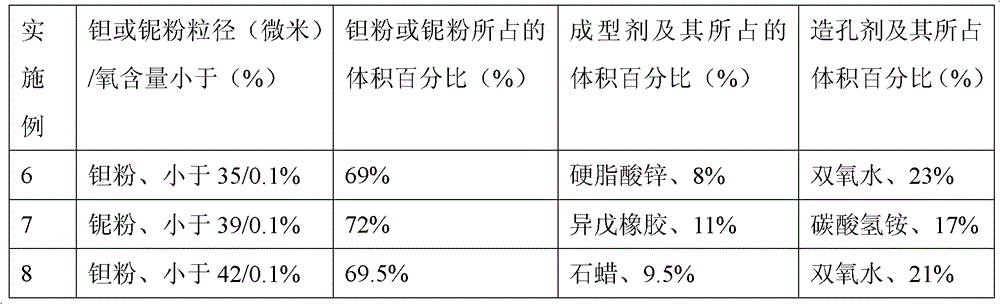
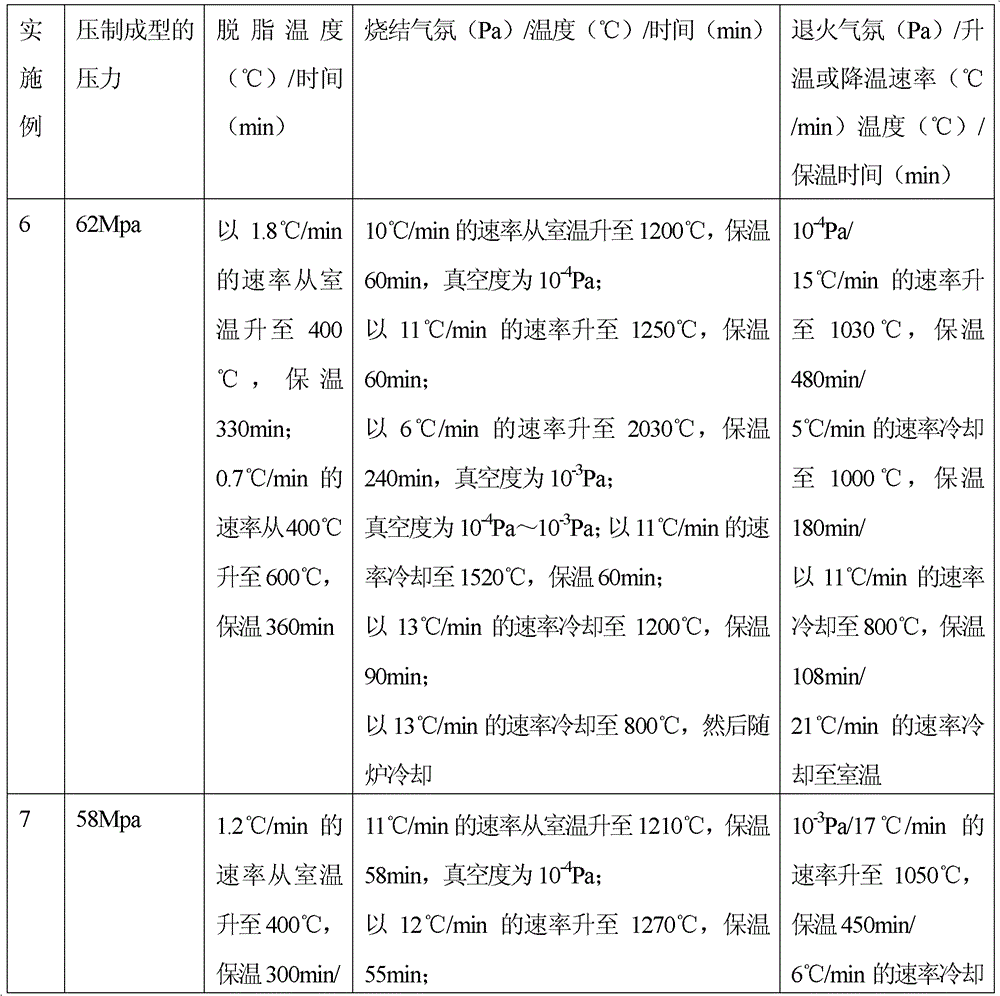
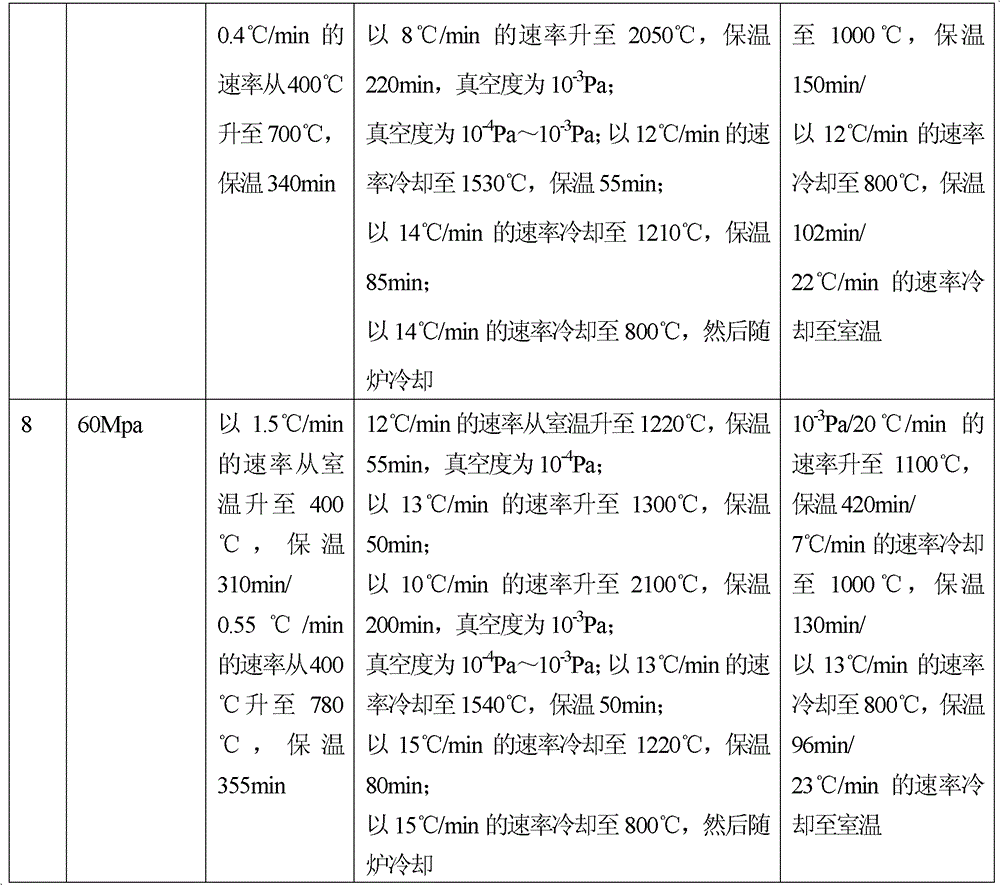
[0031] Embodiment 1: Weigh zinc stearate, average particle diameter is less than 43 micron oxygen content is less than 0.1% tantalum powder and hydrogen peroxide mix uniformly, wherein zinc stearate accounts for 11%, hydrogen peroxide accounts for 18%, tantalum powder accounts for 71%, All are calculated by volume percentage. Compression molding: Add the above mixed powder into an injection molding machine and press it into a polyurethane foam (pore diameter 0.48-0.89mm, density 0.015g / cm2) at 82Mpa 3 ~0.035g / cm 3 , hardness greater than 50°) in molding. Degreasing treatment: vacuum degree 10 -4 Pa, from room temperature to 400°C at a heating rate of 2.0°C / min, and hold for 320 minutes; then raise the temperature from 400°C to 700°C at a heating rate of 0.5°C / min, and hold for 350 minutes. Vacuum sintering: sintering in a vacuum furnace, the sintering temperature is 2000°C, the heat preservation is 2 hours, and the vacuum degree is 10 -4 Pa, the sintering process is filled...
Embodiment 2
[0033] Embodiment 2: Weigh stearic acid, tantalum powder and ammonium bicarbonate with an average particle size less than 43 microns and an oxygen content of less than 0.1% and mix evenly, wherein stearic acid accounts for 7%, ammonium bicarbonate accounts for 25%, and tantalum powder accounts for 68% %, all in volume percentage. Compression molding: Add the above mixed powder into an injection molding machine and press it into a polyurethane foam (pore diameter 0.48-0.89mm, density 0.015g / cm2) at 87Mpa 3 ~0.035g / cm 3 , hardness greater than 50°) in molding. Degreasing treatment: vacuum degree 10 -4 Pa, from room temperature to 400°C at a heating rate of 2°C / min, and hold for 300min. Vacuum sintering: sintering in a vacuum furnace, the sintering temperature is 2100°C, the heat preservation is 4 hours, and the vacuum degree is 10 -4 Pa, the sintering process is filled with argon gas protection, the dust and dirt on the surface are removed after the product is taken out, and...
Embodiment 3
[0035] Embodiment 3: Weigh styrene-butadiene rubber, tantalum powder with an average particle diameter of less than 43 microns and an oxygen content of less than 0.1%, and mix evenly with hydrogen peroxide, wherein styrene-butadiene rubber accounts for 12%, hydrogen peroxide accounts for 15%, and tantalum powder accounts for 73%, all with Volume percentage meter. Pressure molding: Add the above mixed powder into an injection molding machine and press it into a polyurethane foam (pore diameter 0.48-0.89mm, density 0.015g / cm2) at 52Mpa 3 ~0.035g / cm 3 , hardness greater than 50°) in molding. Degreasing treatment: vacuum degree 10 -4 Pa, from room temperature to 400°C at a heating rate of 0.3°C / min, and hold for 360min. Vacuum sintering: sintering in a vacuum furnace, the sintering temperature is 2200°C, the heat preservation is 2.5 hours, and the vacuum degree is 10 -3 Pa, the sintering process is filled with argon protection, cooled out of the furnace, dust and dirt on the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com