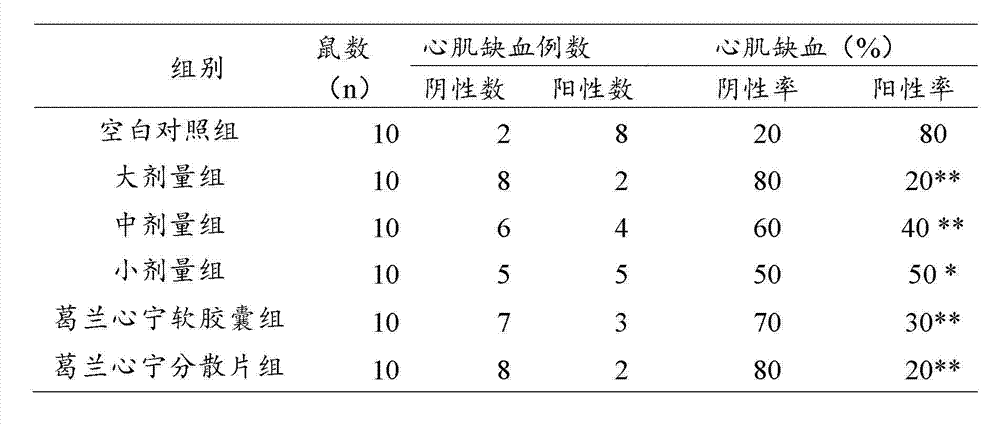
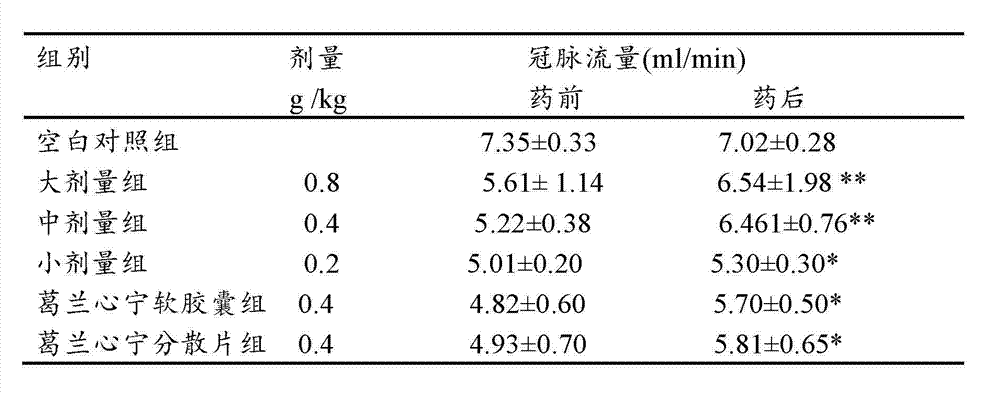
Gelan Xinning soft capsule for treating coronary disease and angina and preparation method thereof
A coronary heart disease angina pectoris and gelatin technology, which is applied in the treatment of coronary heart disease angina pectoris Gelanxinning soft capsules and its preparation field, can solve the problems of complex preparation methods of the raw material kudzu root total flavonoids, long production cycle, and small breakthrough in therapeutic effect, etc. Achieve significant clinical therapeutic economic significance, short production cycle, no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of Chinese patent medicine for treating angina pectoris of coronary heart disease. 1 part of hydrogenated palm oil, 0.2 part of soybean lecithin, 0.01 part of simethicone, 8 parts of gelatin, 3 parts of glycerin, 8 parts of purified water and 0.01 part of ethyl paraben.
[0047] The preparation method of this Chinese patent medicine comprises the following steps:
[0048] (1) Preparation of kudzu root total flavonoids:
[0049] Take Pueraria mirifica, break it into coarse powder, soak it with 60% ethanol aqueous solution for 1 hour, and then reflux for extraction twice, each time with 8 times the weight of the drug, reflux for 2 hours each time, combine the two extracts, Filtrate, concentrate under reduced pressure to an extract with a relative density of 1.16 (60°C heat test), spray dry, grind through a 80-mesh sieve, and obtain the total flavonoids of kudzu root;
[0050] (2) Preparation of hawthorn extract:
[0051] Take hawthorn slices, and use 80% ethanol...
Embodiment 2
[0058] A kind of Chinese patent medicine for treating coronary heart disease and angina pectoris. 0.6 parts of hydrogenated palm oil, 0.4 parts of soybean lecithin, 0.02 parts of simethicone, 12 parts of gelatin, 5 parts of glycerin, 10 parts of purified water and 0.02 parts of ethylparaben.
[0059] The preparation method of this Chinese patent medicine comprises the following steps:
[0060] (1) Preparation of kudzu root total flavonoids:
[0061] Take Pueraria mirifica, break it into coarse powder, soak it with 65% ethanol aqueous solution for 1 hour, and then reflux for extraction twice, each time with a solvent 6 times the weight of the drug, and reflux for 1.5 hours each time, combine the two extracts, Filtrate, concentrate under reduced pressure to an extract with a relative density of 1.28 (60°C heat test), then vacuum-dry, crush and pass through an 80-mesh sieve to obtain the total flavonoids of kudzu root;
[0062] (2) Preparation of hawthorn extract:
[0063] Tak...
Embodiment 3
[0070] A kind of Chinese patent medicine for treating coronary heart disease and angina pectoris. 1 part of hydrogenated palm oil, 0.6 part of soybean lecithin, 0.03 part of simethicone, 25 parts of gelatin, 8 parts of glycerin, 20 parts of purified water and 0.04 part of ethylparaben.
[0071] The preparation method of this Chinese patent medicine comprises the following steps:
[0072] (1) Preparation of Pueraria total flavonoids:
[0073] Take Pueraria mirifica, break it into coarse powder, infiltrate with 75% ethanol aqueous solution for 1 hour, and then reflux for extraction twice, each time with 7 times the amount of drug weight solvent, reflux for 2.5 hours each time, combine the two extracts, Filtrate, concentrate under reduced pressure to an extract with a relative density of 1.25 (thermally measured at 60°C), spray-dry, and crush through a 80-mesh sieve to obtain the total flavonoids of kudzu root;
[0074] (2) Preparation of hawthorn extract:
[0075] Take hawtho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com