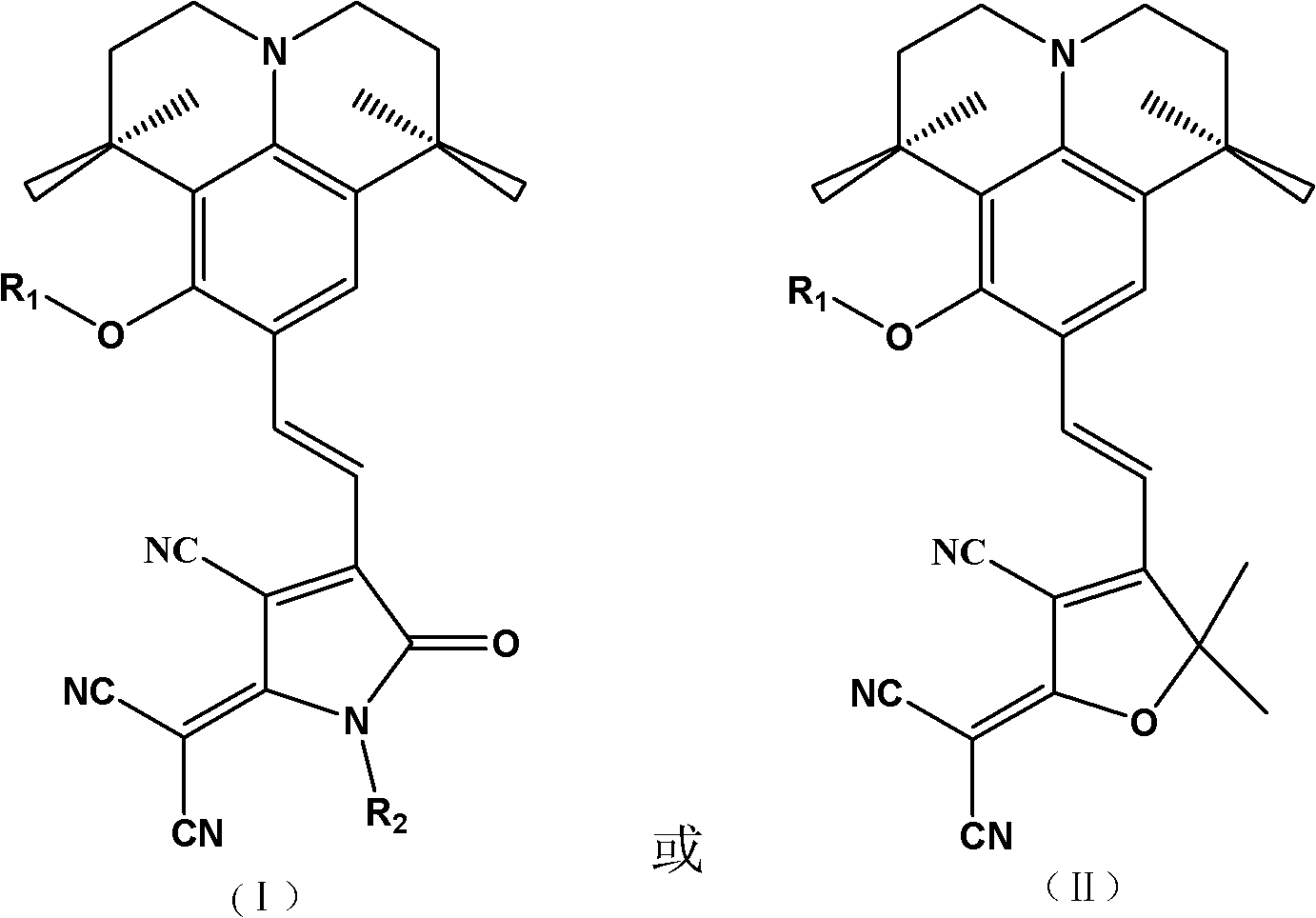
Second-order nonlinear optical chromophore having D-pi-A structure and treating julolidine derivative as donor, and synthetic method and use thereof
A second-order nonlinear and julolidine technology, which is applied to the second-order nonlinear optical chromophore having a D-π-A structure and the synthesis and application fields of a julolidine derivative as a donor, and can Solve the problems of low solubility of polymer base, low polarization efficiency, and large interaction force, and achieve the effects of high preparation yield, reduced interaction force, and high thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0047]
[0048] The synthetic route is as follows:
[0049]
[0050] The synthesis method is:
[0051] 1) 8-bean oxygen group-1,1,7, the synthesis of 7-tetramethyljulonidine-9-formaldehyde (being 1 in the formula)
[0052] Add 2.72g (0.01mol) 8-hydroxy-1,1,7,7-tetramethyljulonidine-9-carbaldehyde, 2.05g (0.012mol) benzyl bromide, and 30mL N,N -Dimethylformamide, in N 2 Add 1.7g (0.012mol) of dried anhydrous potassium carbonate under protection, and react at 120°C for 24 hours. After the reaction is completed, cool down, remove potassium carbonate by filtration, pour the filtrate into water to obtain a dark yellow solution, add hydrochloric acid to adjust the pH to neutral nature, extracted three times with ethyl acetate, combined the organic phases, dried overnight with anhydrous magnesium sulfate, filtered, removed the ethyl acetate solvent by rotary ...
Embodiment 2
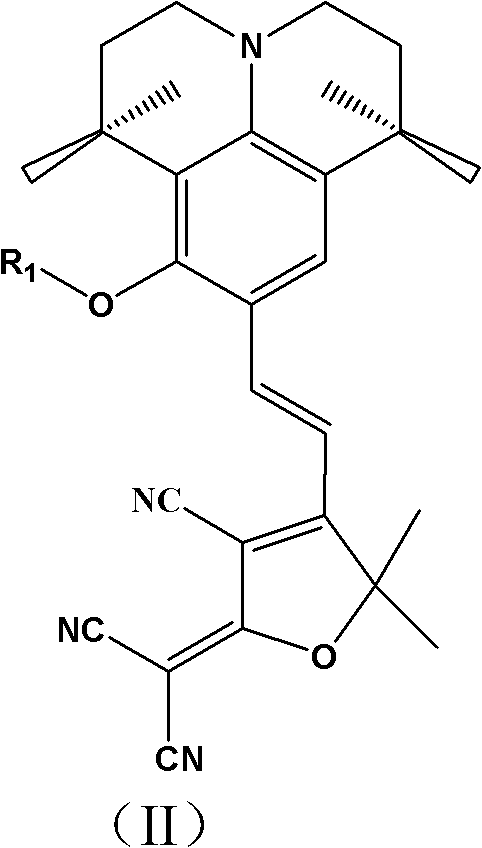
[0063] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0064]
[0065] The synthetic route is as follows:
[0066]
[0067] The synthesis method is:
[0068] 1) The synthesis of 8-beanoxy-1,1,7,7-tetramethyljulonidine-9-carbaldehyde (1 in the formula) is the same as in Example 1.
[0069] 2) Synthesis of 2-amino-1,1,3-tricyanopropene (2 in the formula)
[0070] Add 6.6g (0.1mol) of malononitrile to KOH solution in ethanol at 0°C (2.8g / 20mL, it can be dissolved in 15 minutes), stir at room temperature for about 0.5 hours, a large amount of white precipitates are produced, and after reflux for 0.5 hours, The product is slightly pink, cooled and filtered, rinsed with cold ethanol, dissolved in 100mL of water, added dropwise with concentrated hydrochloric acid to pH=8-9, white precipitate began to precipitate, continued to dropwise added concentrated hydrochloric acid until pH=4, filtered out the precipitate ,...
Embodiment 3
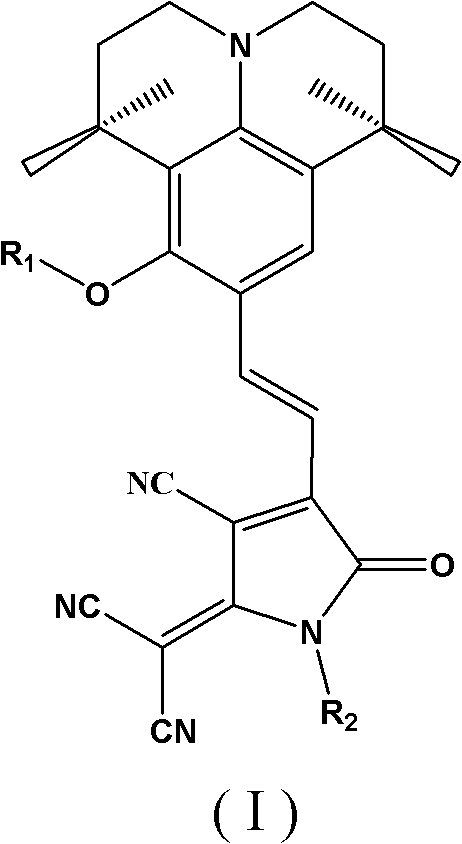
[0081] An organic second-order nonlinear optical chromophore with a D-π-A structure was synthesized as shown below:
[0082]
[0083] The synthetic route is as follows:
[0084]
[0085] The synthesis steps are as follows:
[0086] 1) Synthesis of 8-tert-butyldimethylsilyloxy-1,1,7,7-tetramethyljulonidine-9-carbaldehyde (1 in the formula)
[0087] Add 2.72g (0.01mol) 8-hydroxyl-1,1,7,7-tetramethyljulonidine-9-carbaldehyde, 1.8g (0.012mol) tert-butyldimethyl Chlorosilane, 0.82g (0.012mol) imidazole and 30mL N,N-dimethylformamide, reacted at 30°C for 24 hours under sealed conditions, poured into 500ml of water, extracted three times with ethyl acetate, combined the organic phases, Dry overnight with anhydrous magnesium sulfate, filter, remove the ethyl acetate solvent by rotary evaporation, and separate by column chromatography (with 200-300 mesh silica gel as the stationary phase, and a mixture of n-hexane and acetone as the mobile phase, wherein: n-hexane and acetone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com