Novel high-temperature beta-glucosidase, its coding gene and application
A coding and application technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of low β-glucosidase activity, unsatisfactory yield, physical and chemical properties, and catalytic efficiency, and achieve the effect of strong tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1, the isolation of β-glucosidase and its coding gene
[0094] Using metagenomic technology, the β-glucosidase-positive clone Y8001 was screened from the termite intestinal metagenome system by conventional methods, and the plasmid DNA of this clone was extracted for 454 high-throughput sequencing. After the sequences were spliced, a relatively complete fosmid fragment could be obtained sequence. Search ORF with DNAStar software, search GenBank database with Blastn of NCBI (http: / / www.ncbi.nlm.nih.gov), obtain the coding gene of β-glucosidase, this gene has SEQ ID No.1 in the sequence listing The nucleotide sequence was named bgl1. From the 5' end of SEQ ID No.1 in the sequence listing, the 1st to 1368 nucleotides are the open reading frame (Open Reading Frame, ORF) of bgl1, from the 1st-3rd core of the 5' end of SEQ ID No.1 The nucleotide is the start codon ATG of the bgl1 gene, and the 1366th to 1368th nucleotides from the 5' end of SEQ ID No. 1 are the s...
Embodiment 2
[0096] Example 2, expression of bgl1 in Escherichia coli
[0097] 1. Construction of recombinant expression vector
[0098] The predicted β-glucosidase ORF coding gene was amplified by PCR from the fosmid-positive clones screened above, and the forward primer used was: 5'CGCGGATCCATGGAAAAATATGTGTTT 3'(SEQ ID NO: 3), and BamH was added to its 5' end I recognition site: GGATCC; the reverse primer is 5' CCGCTCGAGTTACCAA TCGGCAAAACC 3' (SEQ ID NO: 4), and the Xho I recognition site: CTCGAG is added to its 5' end.
[0099] After the PCR product was purified, it was digested with BamH I and Xho I, and the DNA fragment was recovered by using the Axygen PCR product column recovery kit, and the DNA fragment was mixed with the recovered vector pET-28a(+) ( Novagen) was ligated overnight at 16°C with T4 DNA ligase to obtain the recombinant expression vector pET28a(+)-bgl1. There is a His tag (6×His-Tag) provided by the expression vector at the N-terminus of the expression product to fa...
Embodiment 3
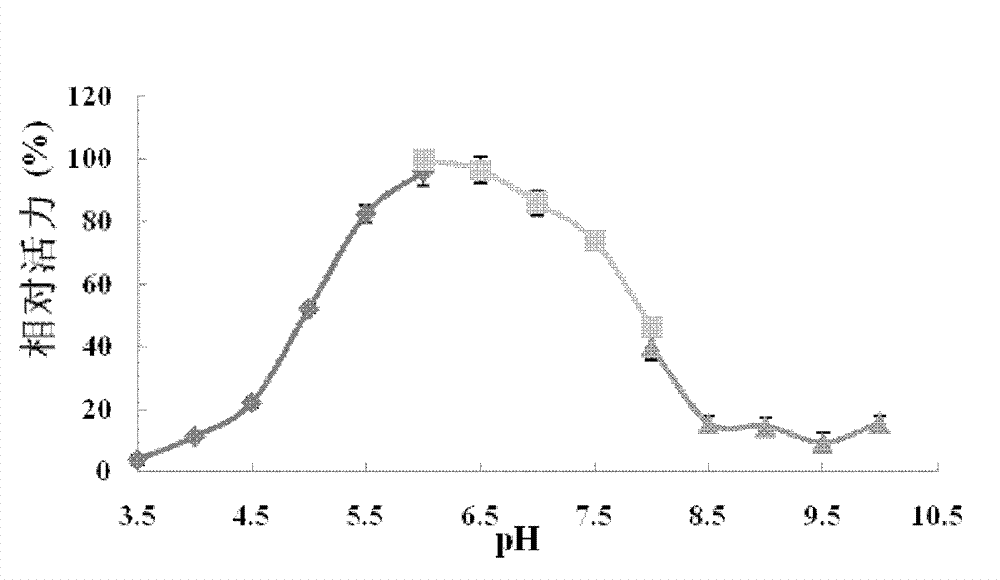
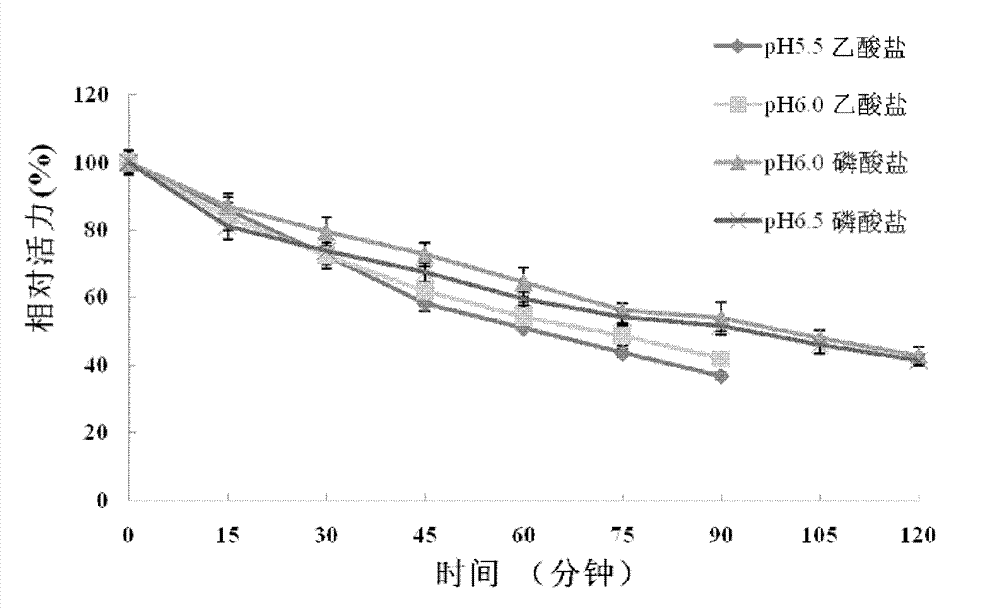
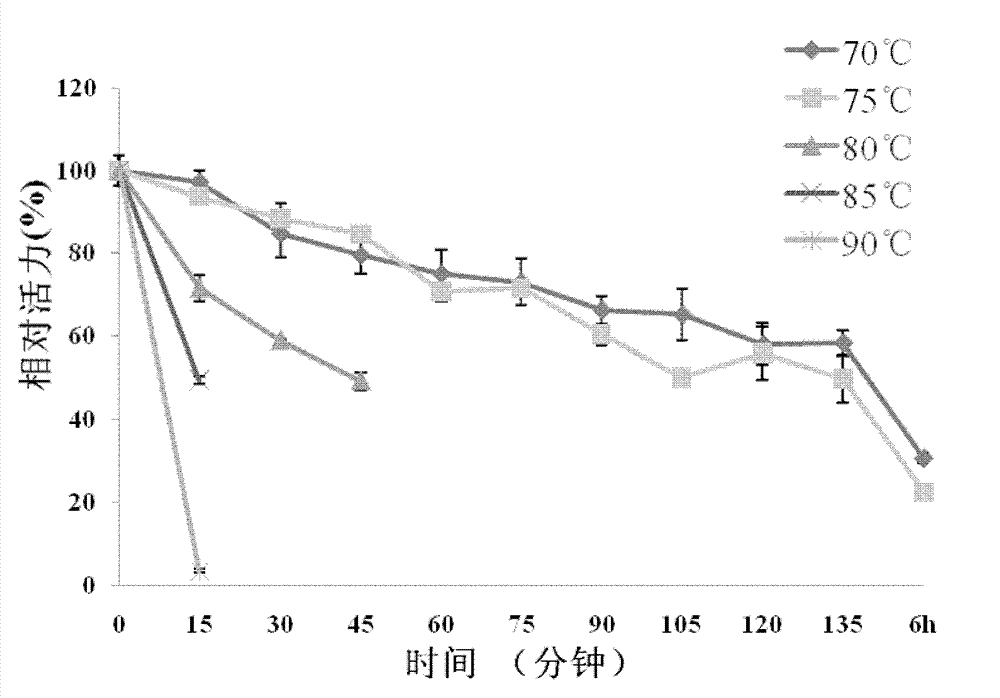
[0108] Example 3. Analysis of recombinant Bgl1 proteolytic properties
[0109] Use p-nitrophenyl-β-D-glucoside (pNPG) as the substrate to detect the enzyme activity and physical and chemical properties of β-glucosidase, the specific operation is as follows:
[0110] (1) p-nitrophenol (pNP) standard curve preparation
[0111] Take a PCR plate arranged in 8*3, add the solution according to Table 4, a total of 8 groups, and each group has 3 parallel repetitions.
[0112] Table 4
[0113]
[0114] The pNP concentration was 10 mg / ml. Add 100 μl 1M NaCO3 to each standard sample in the above table to stop the reaction and develop color, pipette 100 μl into the microplate plate, measure the light absorption at 405 nm with a microplate reader, and the standard sample number 0 is the blank control. The marking line was prepared after subtracting the blank from each sample value.
[0115] (2) Determination of standard enzyme activity
[0116] In a 100μl reaction system, add 50μl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com