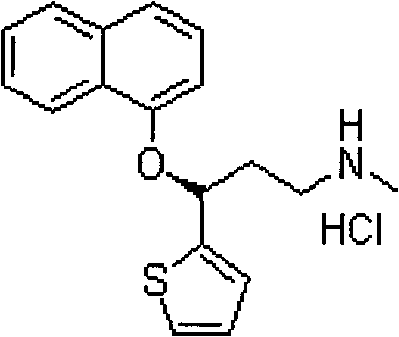
Duloxetine hydrochloride enteric capsules and preparation method thereof
A duloxetine hydrochloride and loxetine enteric technology, which is applied in the field of enteric-coated capsules containing duloxetine hydrochloride and its preparation, can solve the problems of difficult control of industrial production, long preparation time, cumbersome process, etc., and achieve The effects of good release and bioavailability, uniform drug content, and simplified process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
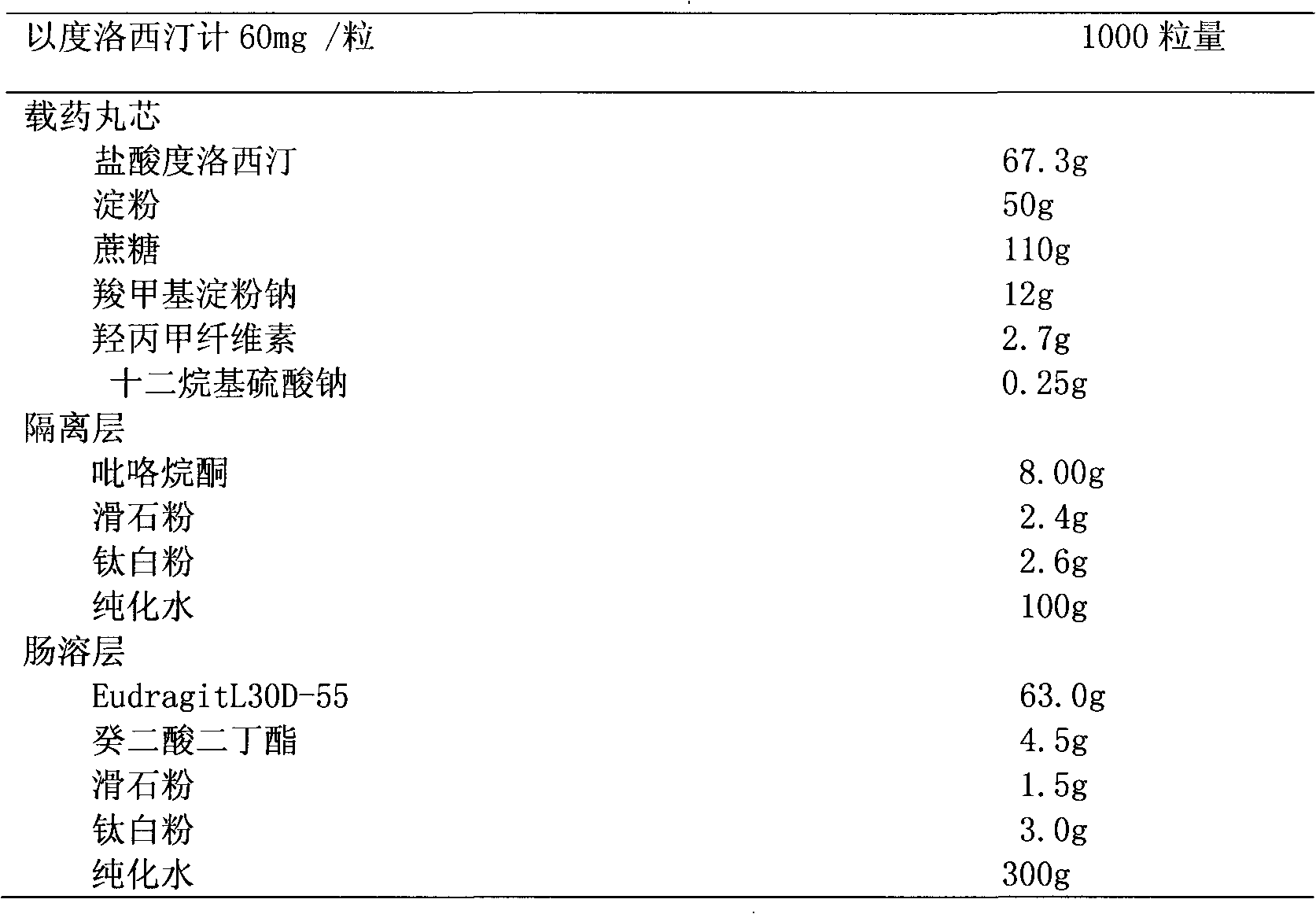
[0057] Duloxetine hydrochloride is air-pulverized, and the particle size distribution range is: 99% of the diameter is not greater than 50 μm, 90% of the diameter is not greater than 35 μm, 50% of the diameter is not greater than 20 μm, and 5% of the diameter is not greater than 10 μm. The prescription quantity of duloxetine hydrochloride, sucrose, microcrystalline cellulose, cross-linked PVP, and sodium lauryl sulfate is mixed evenly, and 5% hypromellose aqueous solution is used as a binder to prepare a soft material, which is extruded and spheronized Prepare drug-loaded pellet cores with a diameter of 0.425 mm to 1.4 mm for later use. Preparation of the coating solution for the isolation layer: add the prescribed amount of hypromellose to the prescribed amount of water, then add polyethylene glycol and stir to dissolve for later use. Add talcum powder and titanium dioxide, stir evenly, stir at a slow speed for half an hour, and coat with a fluidized bed under continuous stir...
Embodiment 2
[0061] Airflow crush duloxetine hydrochloride, the particle size distribution range is: 99% diameter is not greater than 40 μm, 90% diameter is not greater than 25 μm, 50% diameter is not greater than 10 μm, 5% diameter is not greater than 5 μm, spare, weighed according to prescription 2 Prescription amount of duloxetine hydrochloride, sodium carboxymethyl starch, sucrose, starch, sodium lauryl sulfate mixed evenly, 5% hypromellose as binder, preparation of soft material, prepared by extrusion spheronizer Drug-loaded pill cores with a diameter of 0.425 mm to 1.4 mm are used for later use. Preparation of the coating solution for the isolation layer: add the prescribed amount of pyrrolidone (PVP) to the prescribed amount of water, stir to dissolve and set aside, then add talcum powder and titanium dioxide, stir evenly, stir at a slow speed for half an hour, continue to stir, and use a flow Chemical bed for coating. Enteric-coated layer preparation: Weigh the prescription quanti...
Embodiment 3
[0065] Duloxetine hydrochloride is air-pulverized, and the particle size distribution range is: 99% of the diameter is not greater than 40 μm, 90% of the diameter is not greater than 25 μm, 50% of the diameter is not greater than 10 μm, and 5% of the diameter is not greater than 5 μm. For standby, take according to prescription 3 Prescription amount of duloxetine hydrochloride, sucrose, sodium carboxymethyl starch, and starch are mixed evenly, and 5% hypromellose aqueous solution is used as a binder to prepare a soft material, which is prepared by an extrusion spheronizer into a diameter of 0.425 mm to 1.4 mm drug-loaded pill core, spare. Preparation of the coating solution for the isolation layer: add the prescribed amount of pyrrolidone to the prescribed amount of 50% ethanol aqueous solution (mass ratio), then add polyethylene glycol and stir to dissolve it for later use, then add talcum powder and titanium dioxide, stir evenly, and stir at a slow speed For half an hour, un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com