Catalyst for dimethyl ether preparation through low-temperature methanol dehydration and preparation method as well as applications thereof
A low-temperature methanol and catalyst technology, applied in the field of catalysts for dimethyl ether synthesis, can solve the problems of unsatisfactory high temperature resistance performance and activity of the catalyst, insufficient ability of the catalyst to process raw materials, and inability to better conform to green chemical industry, etc. Environmental friendliness and economy, easy to obtain preparation method, good environment, good low temperature reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of macroporous sulfonic acid cation exchange resin
[0041]a. Polymerization: In a 2L stainless steel polymerization kettle, add 1200g of water and 2.5g of polyvinyl alcohol, heat and stir to completely dissolve the additives in the kettle. Heat up to 60°C, add 180g styrene (styrene content ≥ 99%), 130g divinylbenzene (divinylbenzene content 50%), 120g32 # White oil, an organic phase mixture composed of 2.6 g of benzoyl peroxide, stirred and heated to 86° C., and reacted at a constant temperature for 10 hours. The reaction product was filtered and dried at room temperature (moisture content ≤ 3%) to obtain 400 g of dry polymer white balls, of which 85.2% were polymers with particle sizes ranging from 0.3 mm to 1.0 mm.
[0042] b. Purification of pore structure: Take 100 g of dry white balls with a particle size of 0.3 mm to 1.0 mm and place them in a Soxhlet extractor. Extract for 2.5 hours. Then move the white ball into another 1000mL flask, add 500mL...
Embodiment 2
[0060] 1. During the preparation of the resin catalyst, the steps of polymerization, pore structure purification, sulfonation, filtration and drying are the same as those in Example 1.
[0061] 2. Fluorination of macroporous sulfonic acid resin:
[0062] 1) Load the dried macroporous sulfonic acid resin into the exchange column replaced by nitrogen, and pass through the fluorine-nitrogen mixed gas with a fluorine content of 7%, and the space velocity of the fluorine-nitrogen mixed gas is 20h -1 , the ventilation time is 15h;
[0063] 2) Replace the exchange column with nitrogen again after fluorination.
[0064] 3. Complexation loading AlCl on the fluorinated macroporous sulfonic acid resin 3 The steps are the same as in Example 1.
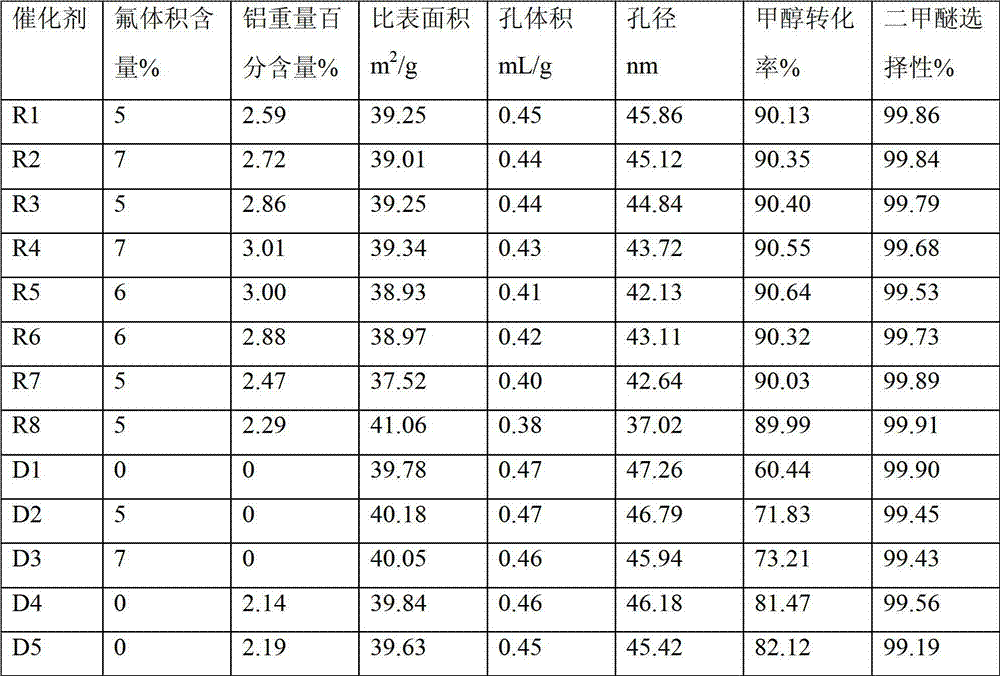
[0065] Through the above steps, the target catalyst R2 is finally obtained. Catalyst specific surface area (determined by BET method), pore volume and pore diameter are respectively: 39.01m 2 / g, 0.44mL / g, 45.12nm. The weight percent content...
Embodiment 3
[0069] 1. During the preparation of the resin catalyst, the steps of polymerization, pore structure purification, sulfonation, filtration and drying are the same as those in Example 1.
[0070] 2. The fluorination step of the macroporous sulfonic acid resin is the same as in Example 1.
[0071] 3. Complexation loading AlCl on the fluorinated macroporous sulfonic acid resin 3 : Add 100g of fluorinated resin, 160g of absolute ethanol and 4.5g of anhydrous aluminum chloride into a three-necked flask, stir and control the reaction temperature to 78°C with a constant temperature water bath, and the reaction time is 4.5h.
[0072] Through the above steps, the target catalyst R3 is finally obtained. Catalyst specific surface area (determined by BET method), pore volume and pore diameter are: 39.25m 2 / g, 0.44mL / g, 44.84nm. The weight percent content of aluminum in the catalyst is 2.86%.
[0073] The application mode of catalyst is consistent with embodiment 1.
[0074] It was de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com