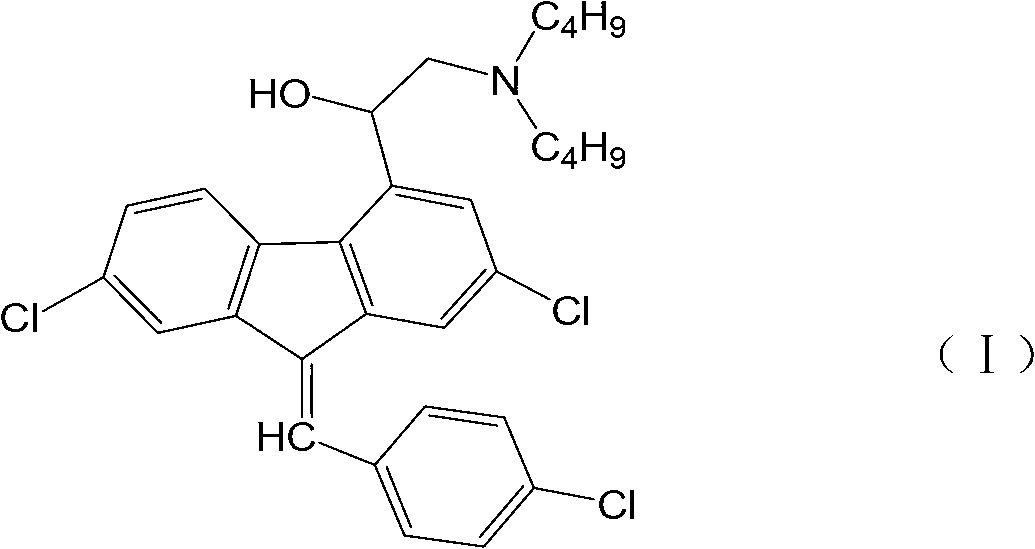
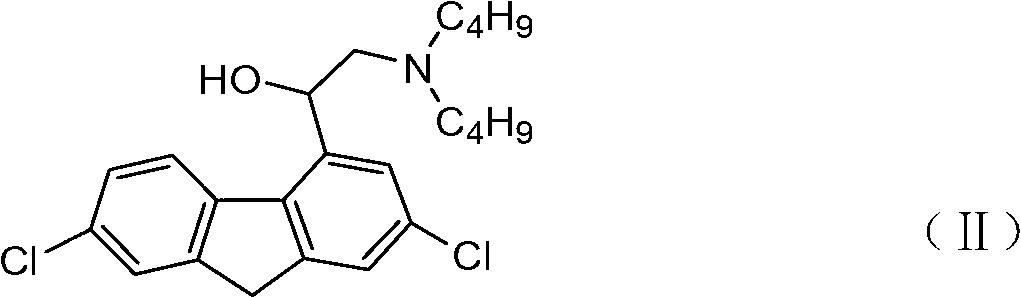
Preparation method for 2-dibutylamido-1-1(2,7- dichloro-9H-fluorine-4base)-ethanol
A dibutylamine-based, dichlorofluorene technology, applied in the field of chemical pharmacy, can solve problems such as reducing production cost, difficulty in filtration, shortening production cycle, etc., so as to improve product yield and product quality, accelerate the process of amination reaction, reduce Effect of impurity generation amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
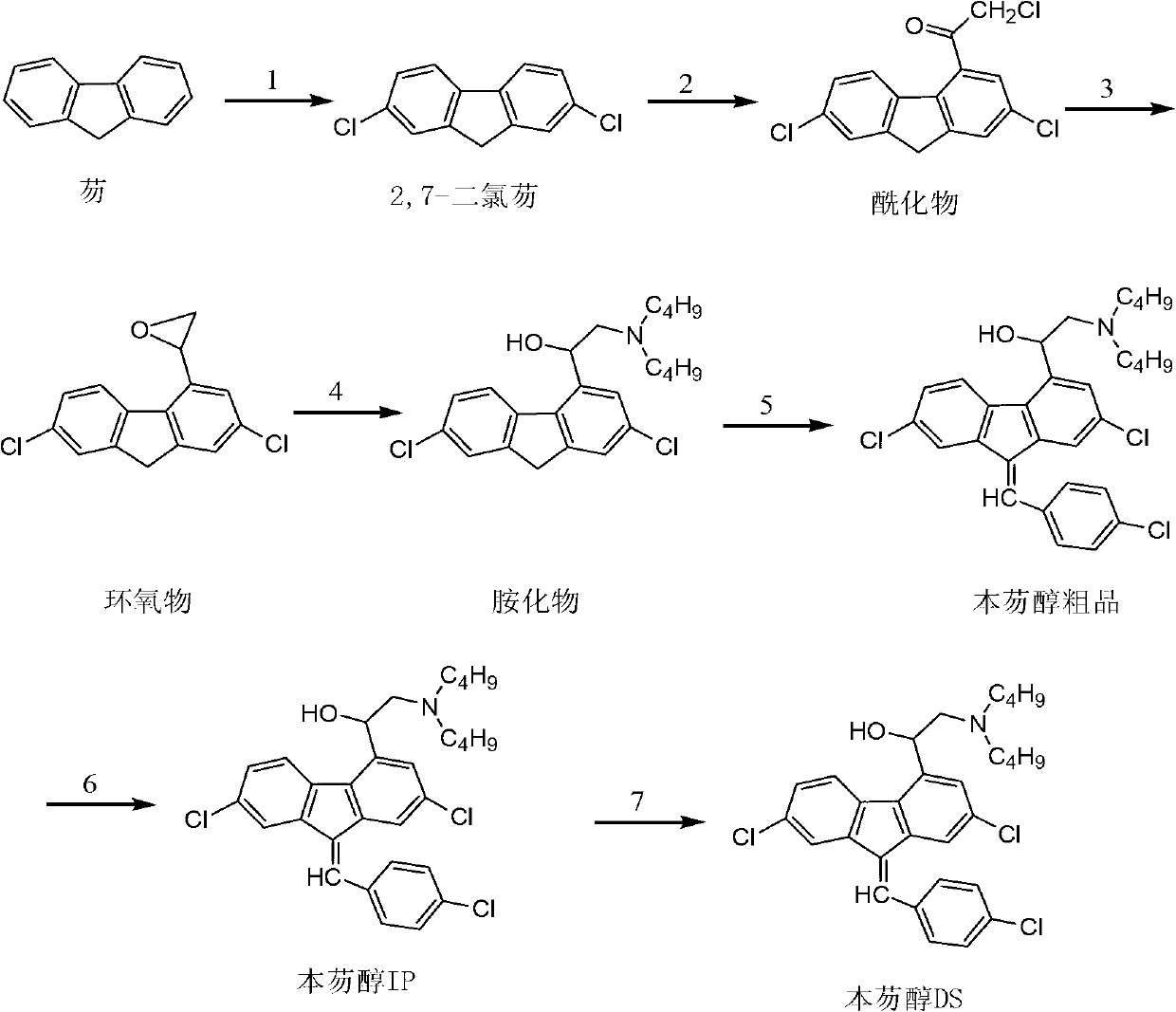
[0041] Example 1 Preparation of 2-dibutylamino-1-(2,7-dichloro-9H-fluoren-4 base)-ethanol
[0042] 1. Weigh 15.1g of chloride (2,7-dichlorofluorene), dissolve it in dichloromethane, control the internal temperature at -22~-18°C, add 12.6g of aluminum trichloride and chloroacetyl chloride dropwise 9.1 g was dissolved in a solution of 31 ml of dichloromethane. After the reaction was completed, the reaction solution was poured into a 10% aqueous solution of hydrochloric acid, allowed to stand for layers, and the organic layer was washed with water, and the dichloromethane in the organic layer was distilled off. methane to obtain a distilled raffinate containing acylate; add ethanol to continue distillation to remove the remaining dichloromethane; add 75ml of ethanol.
[0043] 2. Control the internal temperature at -5-5°C. Within 2.5 hours, add 1.3g of sodium borohydride to the ethanol containing acylate prepared in step 1, keep the temperature for 30-60 minutes, and then add 41.5...
Embodiment 2
[0046] Example 2 Preparation of 2-dibutylamino-1-(2,7-dichloro-9H-fluoren-4 base)-ethanol
[0047] 1. Weigh 15.1g of 2,7-dichlorofluorene, dissolve it in dichloromethane, control the internal temperature at 0-5°C, add dropwise 13.0g of aluminum trichloride and 9.1g of chloroacetyl chloride into 31ml of dichlorofluorene In the solution of methyl chloride, after the reaction is completed, the reaction solution is poured into the aqueous hydrochloric acid solution with a concentration of 15%, and the layers are left to stand, and the organic layer is washed with water, and the dichloromethane in the organic layer is distilled off to obtain acyl chloride containing The distilled raffinate of the compound; Add methanol to continue distillation to remove the remaining dichloromethane; Add 65ml of methanol.
[0048] 2. Control the internal temperature at -5-5°C. Within 2 hours, add 1.3 grams of sodium borohydride to the methanol containing acylate prepared in step 1, and then add 33....
Embodiment 3
[0051] Example 3 Preparation of 2-dibutylamino-1-(2,7-dichloro-9H-fluoren-4-yl)-ethanol
[0052] 1. Weigh 15.1g of 2,7-dichlorofluorene, dissolve it in dichloromethane, control the internal temperature at 0-5°C, add dropwise 12.0g of aluminum trichloride and 10.0g of chloroacetyl chloride into 31ml of dichlorofluorene In the solution of methyl chloride, after the reaction is completed, the reaction solution is poured into the aqueous hydrochloric acid solution with a concentration of 10%, and the layers are left to stand, and the organic layer is washed with water, and the dichloromethane in the organic layer is distilled off to obtain acyl chloride containing The distilled raffinate of compound; add ethanol to continue distillation, remove remaining dichloromethane; add 65ml ethanol.
[0053] 3. Control the internal temperature at -5 to 5°C, add 3.4g of potassium borohydride in portions to the ethanol containing the acylate prepared in step 1, and then add 16.6g of di-n-butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com