Substituted pyridine-2-ketone compounds and preparation method thereof
A ketone compound and compound technology, which are applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of less derivatives of pyridine-2-one compounds, less synthesis methods, etc., and achieve high yield and low price. , good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
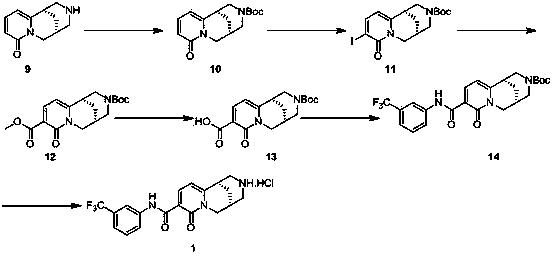
[0054]
[0055]1) Compound 9 (50 g, 0.26 mol) was dissolved in tetrahydrofuran (600 mL), sodium carbonate (32.9 g 0.31 mol) was added, and di-tert-butyl carbonate (67.6 g, 0.31 mol) was added dropwise at 0°C ). After the dropwise addition was completed, the reaction solution was stirred at 30° C. for 16 hours. 200 milliliters of water were added to the reaction solution, extracted 3 times with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain a crude product, which was washed with petroleum ether to obtain compound 10 (69.5 g, yield: 91%). ESI-MS: 291.4 [M+H] + , 1 H NMR (DMSO-d6, 400 MHz) d 7.32 (m, 1H), 6.20-6.12 (m, 2H), 4.20-3.90 (m, 3H), 3.61 (dd, J = 6.4 Hz, 15.6 Hz, 1H), 3.12-2.94 (m, 3H), 2.40-2.35 (m, 1H), 1.95-1.85 (m, 2H), 1.20 (d, J= 47.6 Hz, 9H).
[0056] 2) Compound 10 (5.0 g, 17.2 mmol) and silver sulfate (5.4 g, 17.2 mmol) were dissolved in dichloromethane (150 mL), and iodine (4.4 g, 17....
Embodiment 2
[0062]
[0063] 1) Dissolve compound 13 (67 mg, 0.2 mmol) in 2 mL of dichloromethane, add triethylamine (40 mg, 0.4 mmol), 2,4-difluorobenzylamine (34 mg, 0.24 mmol), respectively, 1-Hydroxybenzotriazole (32 mg, 0.24 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (46 mg, 0.24 mmol), reaction solution Stir at 30°C for 16 hours. The reaction solution was concentrated to obtain crude product 15, which was directly used in the next reaction.
[0064] 2) Dissolve the crude product 15 in methanol (2 mL), add 2 mL of methanolic hydrochloric acid solution (4 mol / L), stir the reaction solution at 30°C for 16 hours, and purify the reaction solution by preparative high performance liquid phase to obtain compound 2 (28.86 mg, yield: 36.5%). ESI-MS: 360.4 [M+H] + , 1 H NMR (DMSO-d6, 400 MHz) d 10.28 (t, J=6.4 Hz, 1H), 9.79 (brs, 1H), 8.32 (brs, 1H), 8.30 (d, J=7.6 Hz, 1H), 7.15-7.09 (m, 1H), 7.02 (t , J=6.4 Hz, 2H), 6.54 (d, J=7.6 Hz, 1H), 4.60 (qd, J= 6.4 ...
Embodiment 3
[0066]
[0067] 1) Compound 11 (15.0 g, 36 mmol) was dissolved in benzyl alcohol (100 mL), and triethylamine (10.9 g, 108 mmol) and tetrakistriphenylphosphine palladium (4.2 g, 3.6 mmol) were added sequentially. Then carbon monoxide gas (45 psi) was introduced, the reaction solution was stirred at 65°C for 24 hours, then cooled to room temperature, the reaction solution was concentrated and purified by column chromatography to obtain white compound 16 (12 g, yield: 78.6%). ESI-MS: 425.0 [M+H] + , 1 H NMR (CD 3 OD-d 4 ) d:8.18 (d, J = 7.6 Hz, 1H), 7.45-7.30 (m, 5H), 6.39 (d, J = 7.6 Hz, 1H), 5.31 (s, 2H), 4.40-4.10 (m, 3H), 3.85 (dd, J = 6.0, 15.6Hz, 1H), 3.30-3.00 (m, 3H), 2.48 (s, 1H), 2.07-1.98 (m, 2H), 1.35-1.10 (m, 9H).
[0068] 2) Dissolve compound 16 (12.0 g, 28.3 mmol) in ethyl acetate (10 mL), add 200 ml of hydrochloric acid / ethyl acetate solution (4 mol / L) dropwise at 0°C, after the addition is complete, the reaction The mixture was reacted at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com