Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
A technology for synchronous detection and meningitis, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganism measurement/testing, etc., can solve the problems of low diagnostic efficiency, uncertain sensitivity, high technical cost, etc., and achieve saving production Cost and detection cost, good sensitivity and specificity, avoid the effect of low specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0040] The present invention is a kit for synchronous detection of 23 types of encephalitis and meningitis pathogens, comprising:
[0041] 1) RT primer (reverse transcription primer RT primer Mix)
[0042] 2) PCR Primer (PCR Primer Mix)
[0043] 3) 25mM magnesium chloride (25mM MgCl2)
[0044] 4) Reverse transcriptase
[0045] 5) DNA polymerase (Taq DNA Polymerase)
[0046] 6) Solution X (Solution X)
[0047] 7) 10x PCR buffer (10x PCR Buffer)
[0048] 8) 5×RT buffer (5x RT buffer)
[0049] 9) Positive Control (Positive Control)
[0050] 11) RNase / DNase-free ultrapure water (DNase / Rnase Free ddH2O)
[0051] 12) Positive control substances (DNA fragments with specific sequences, used for quality control of the entire reaction system)
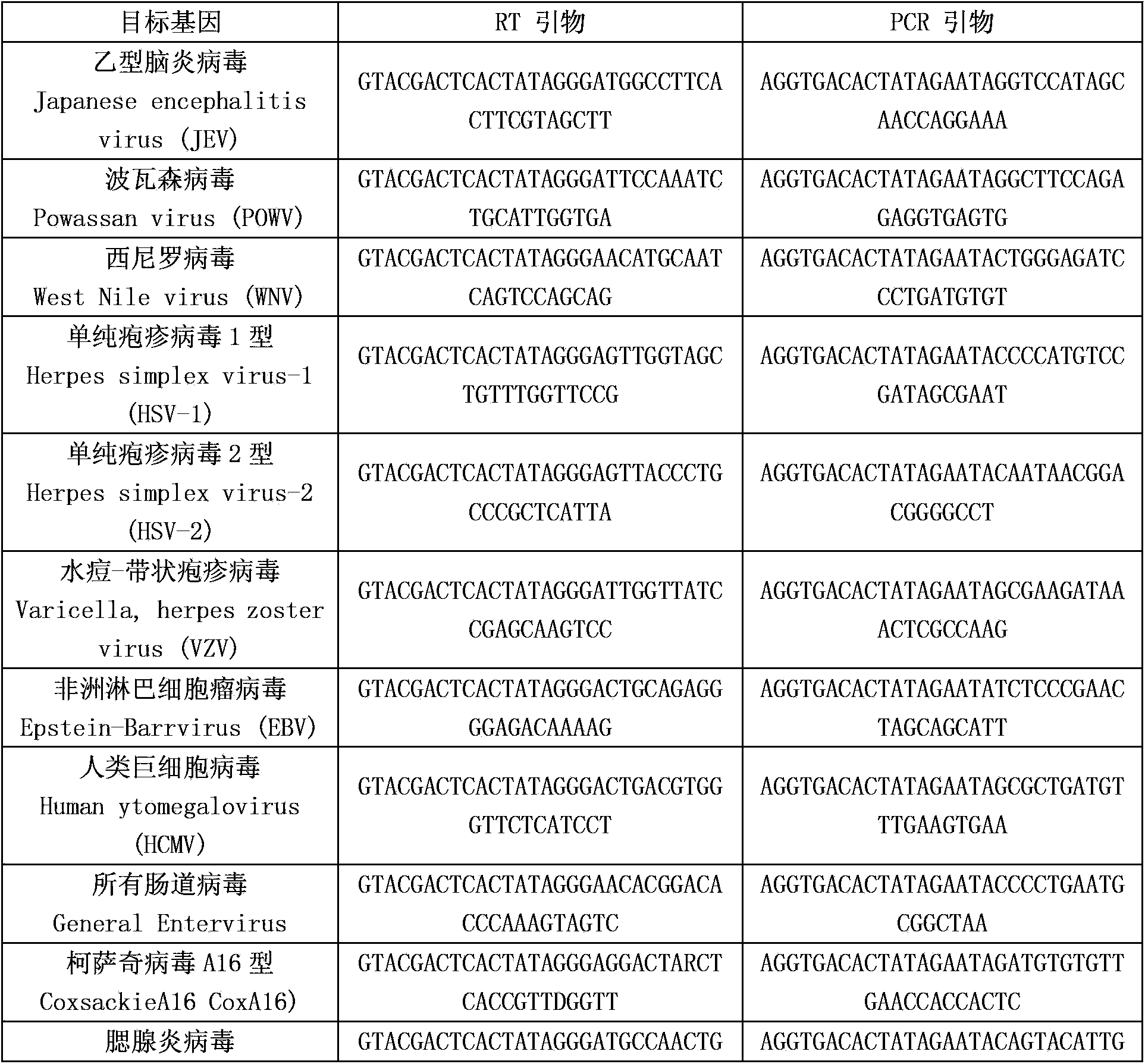
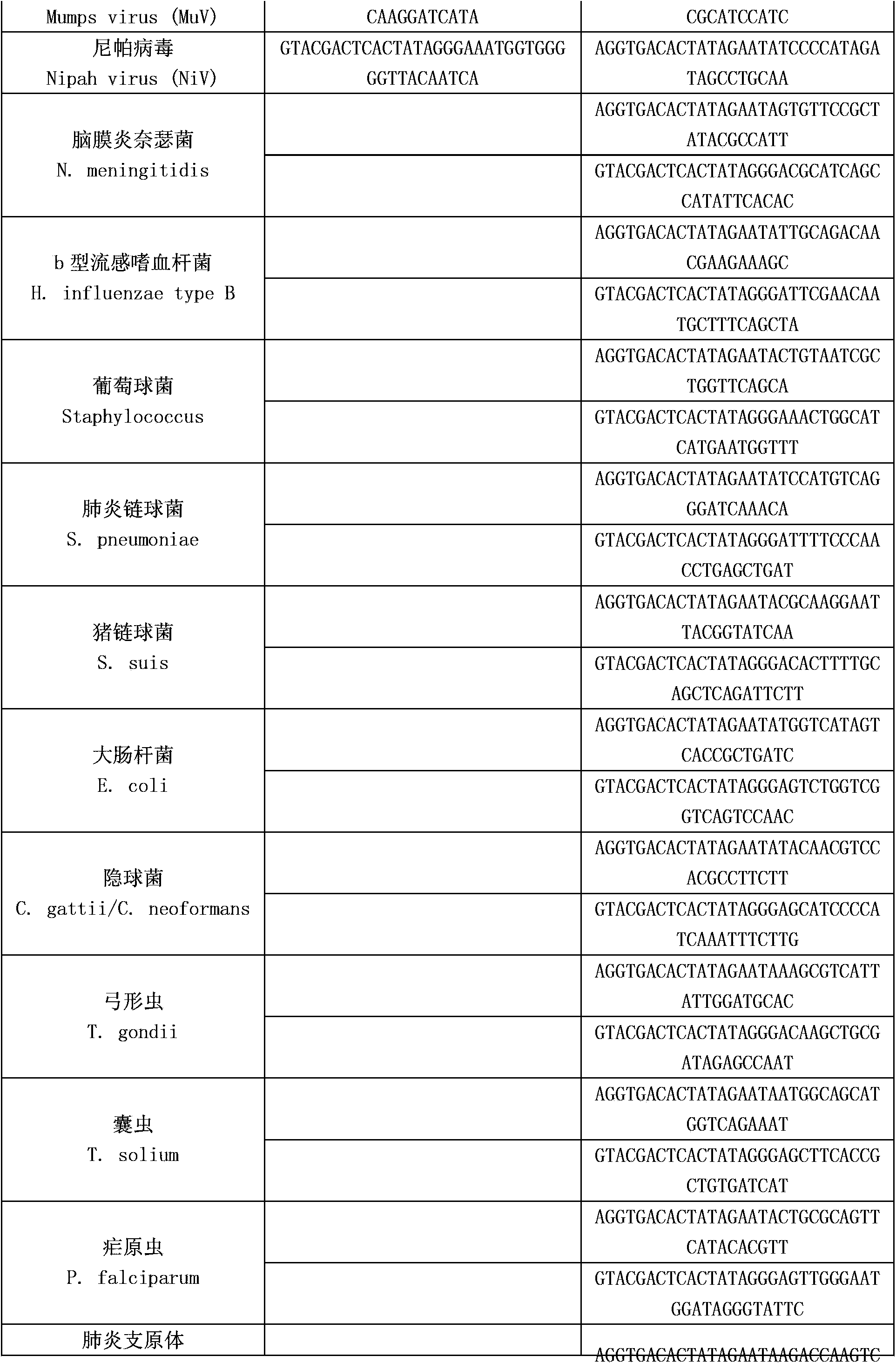
[0052] The above-mentioned reverse transcription primers include the RT amplification primers of twelve kinds of encephalitis and meningitis pathogens in the following table and the RT amplification primers of human RNA internal reference...
specific Embodiment 2
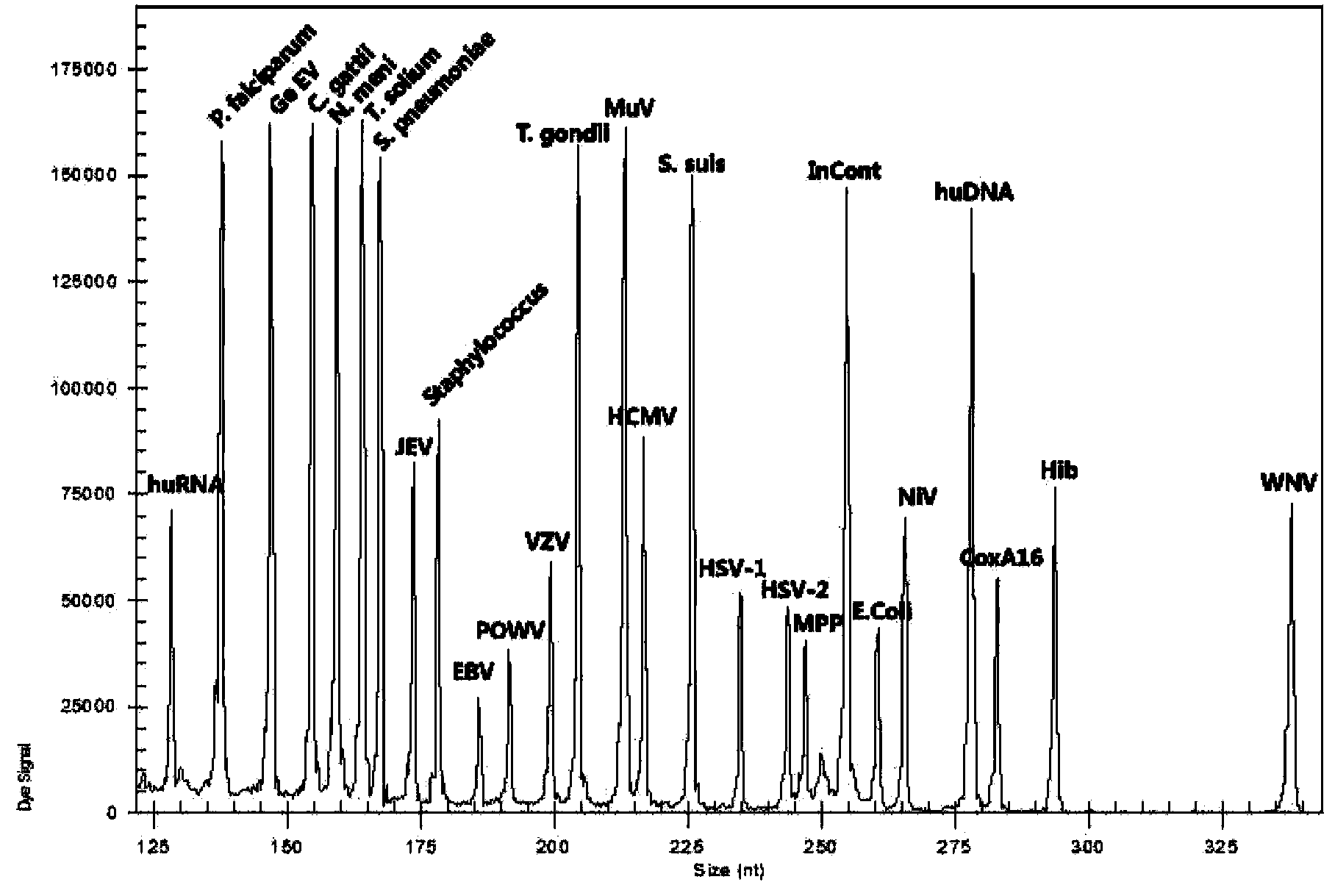
[0062] The invention is a detection method for synchronously detecting 23 kinds of encephalitis and meningitis pathogens, the detected encephalitis and meningitis pathogens include Japanese encephalitis virus, Powassen virus, West Nile virus, herpes simplex virus type 1, simplex Herpesvirus type 2, varicella-zoster virus, African lymphoma virus, human cytomegalovirus, all enteroviruses, coxsackievirus A16, enterovirus 71, mumps virus, Nipah virus, meninges Neisseria, Haemophilus influenzae type b, Staphylococcus, Streptococcus pneumoniae, Streptococcus suis, Escherichia coli, Cryptococcus, Toxoplasma gondii, cysticercosis, Plasmodium, Mycoplasma pneumoniae, etc. (see Table 1), collect patient samples (cerebrospinal fluid, blood, etc.) extract nucleic acid, use patient nucleic acid as a template for reverse transcription and PCR reactions, and finally separate samples by capillary electrophoresis. The specific steps are as follows:
[0063] 1. Production of a kit for the simult...
specific Embodiment 3
[0091] Detection Kit Sensitivity and Specificity Analysis
[0092] Sensitivity analysis: After diluting the positive control according to a certain copy number ratio, it is detected by PCR amplification and capillary electrophoresis until no signal is detected. The copy number is the lowest detection line, which is the sensitivity of the kit. The highest sensitivity can detect 40 copies.
[0093] Specificity analysis: Single-plex PCR amplification is detected as a single peak of the target fragment size by capillary electrophoresis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com