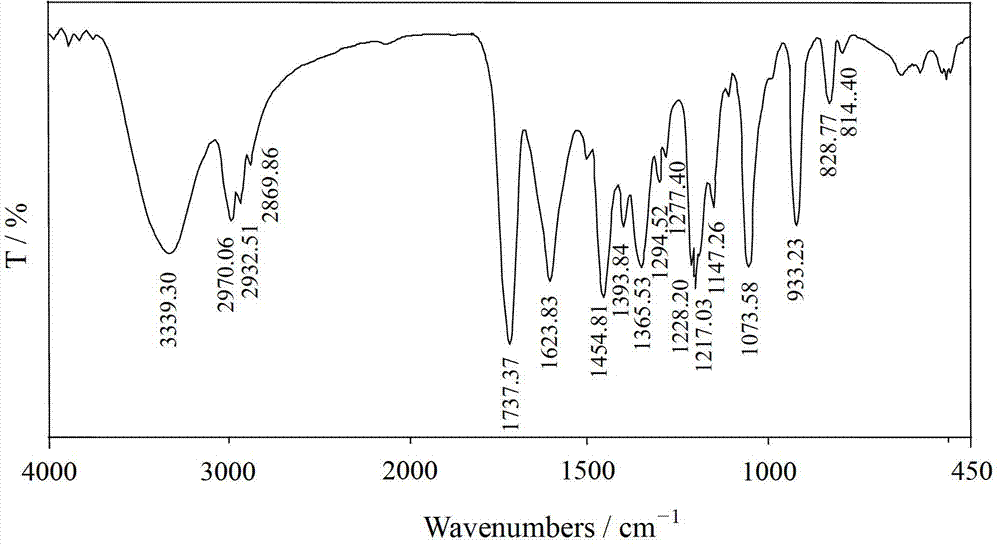
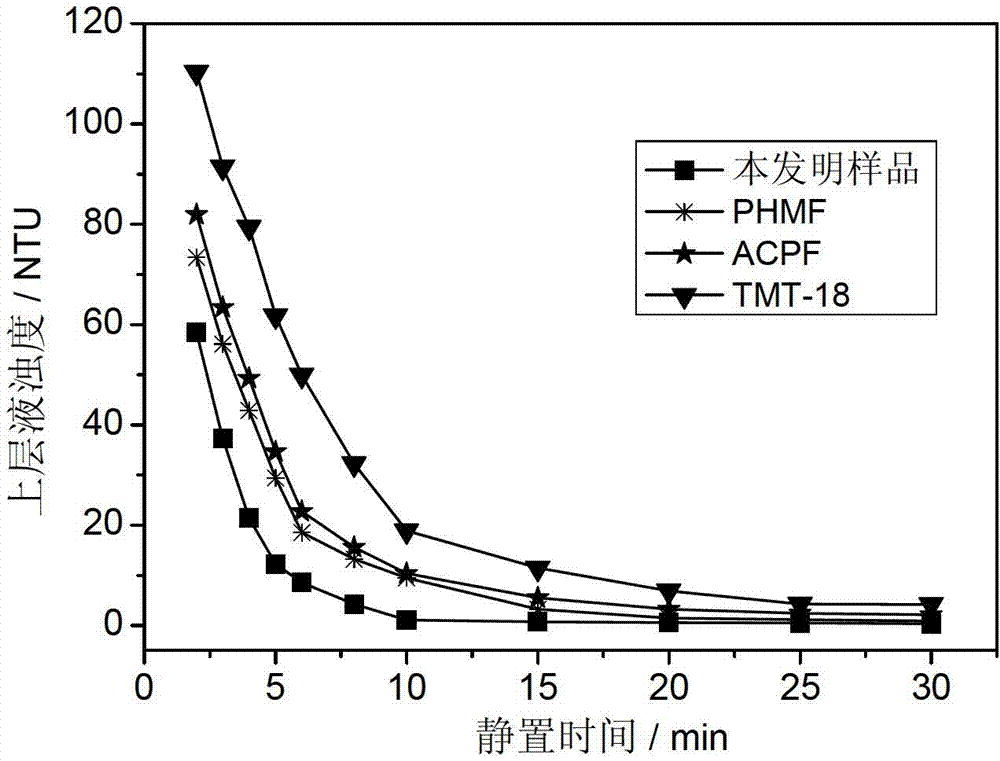
Ethyl polyethylene polyamine polymethacrylate chelating flocculant and preparation method thereof
A technology of poly(ethylene polyamine ethyl ester) and poly(ethylene polyamine) polymethacrylate, applied in the direction of flocculation/precipitation water/sewage treatment, etc. Treatment effect, reduction of volume and moisture content, effect of promoting floc growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
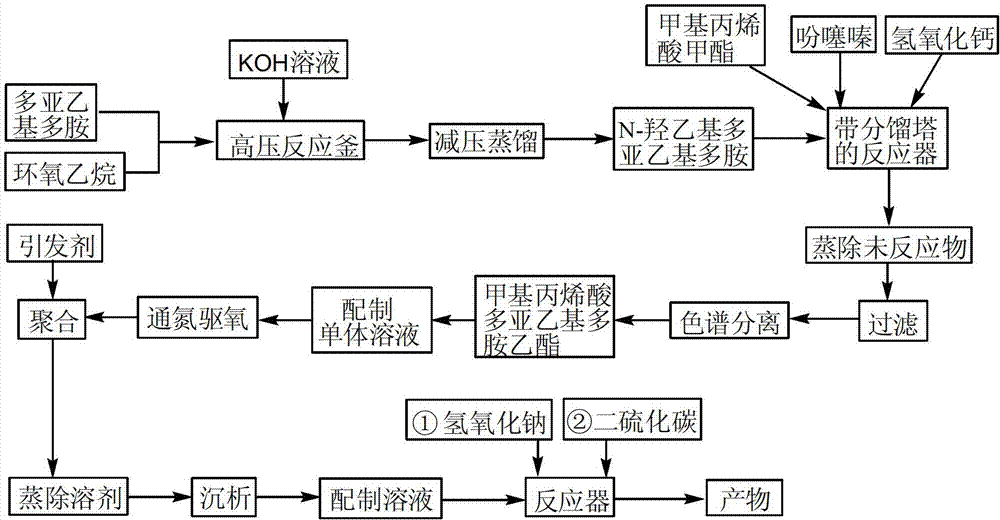
[0032] (1) According to the molar ratio of ethylenediamine and ethylene oxide is 2:1, add 301g of ethylenediamine into a 1L autoclave, and then add 16.4g of potassium hydroxide with a concentration of 10% by mass Solution, heated to boiling, then passed through 110.1g of ethylene oxide, reacted at 160°C and 0.5MPa for 3h, decompressed to 1333Pa, collected fractions at 102-104.5°C, and obtained 242.5g of N-hydroxyethylethylenediamine;
[0033] (2) Add N-hydroxyethylethylenediamine and 467g of methyl methacrylate obtained in step (1) into a 1.5L reactor with a stirrer, a thermometer and a fractionation tower, and then add 25.9g of calcium hydroxide and 6.5g of phenothiazine, reacted at 70°C for 4h, decompressed to 1333Pa to distill off unreacted methyl methacrylate and N-hydroxyethylethylenediamine, then filtered to remove calcium hydroxide, separated and purified by column chromatography to obtain 347.9 g ethylenediamine ethyl methacrylate;
[0034] (3) Dissolve the ethylened...
Embodiment 2
[0039] (1) According to the molar ratio of ethylenediamine and ethylene oxide of 2.05:1, add 308.5g of ethylenediamine into a 1L high-pressure reactor, and then add 21g of potassium hydroxide with a concentration of 10% by mass Solution, heated to boiling, then passed through 110.1g of ethylene oxide, reacted at 170°C and 0.65MPa for 2.5h, decompressed to 1333Pa, collected fractions at 102-104.5°C, and obtained 243.6g of N-hydroxyethylethylenediamine ;
[0040] (2) Add N-hydroxyethylethylenediamine and 515.4g of methyl methacrylate obtained in step (1) into a 1.5L reactor equipped with a stirrer, thermometer and fractionation tower, and then add 29.5g of calcium hydroxide and 7g of phenothiazine, reacted at 80°C for 3.5h, decompressed to 1333Pa to evaporate unreacted methyl methacrylate and N-hydroxyethylethylenediamine, then filtered to remove calcium hydroxide, and purified by column chromatography to obtain 352.2g ethylenediamine ethyl methacrylate;
[0041] (3) Dissolve ...
Embodiment 3
[0045] (1) According to the molar ratio of ethylenediamine and ethylene oxide is 2.1:1, add 316g of ethylenediamine into a 1L high-pressure reactor, and then add 26g of potassium hydroxide solution with a concentration of 10% by mass , heated to boiling, then passed 110.1g of ethylene oxide, reacted at 180°C and 0.8MPa for 2h, decompressed to 1333Pa, collected fractions at 102-104.5°C, and obtained 243.2g of N-hydroxyethylethylenediamine;
[0046] (2) Add N-hydroxyethylethylenediamine and 561.1g of methyl methacrylate obtained in step (1) into a 1.5L reactor equipped with a stirrer, thermometer and fractionation tower, and then add 31.1g of calcium hydroxide and 7.45g of phenothiazine, reacted at 90°C for 3h, decompressed to 1333Pa to evaporate unreacted methyl methacrylate and N-hydroxyethylethylenediamine, then filtered to remove calcium hydroxide, and purified by column chromatography to obtain 349.5g ethylenediamine ethyl methacrylate;
[0047] (3) Dissolve ethylenediami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com