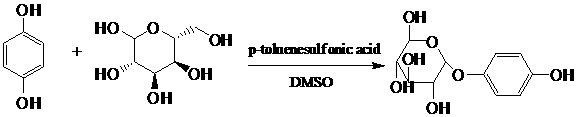
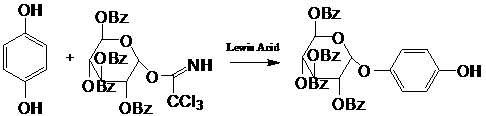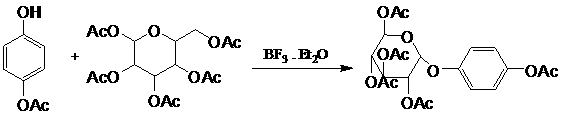Method for synthesis of alpha-arbutin
A synthesis method and technology of arbutin, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of low yield, difficult to achieve production, and single, and achieve industrialized production and purification. The effect of easy and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of High Purity α-Type Intermediate Using Dichloromethane as Solvent
[0042] Under the protection of nitrogen, add 10g of pentaacetylglucose, 5.64g of hydroquinone, 10g of boron trifluoride ether and 50mL of dichloromethane into a 100mL reaction bottle, heat and reflux for 72h, the reaction solution is neutralized by sodium bicarbonate, saturated saline After washing three times, drying over anhydrous magnesium sulfate, and concentrating, 10.2 g of a light brown oil was obtained, and the content of α-isomer was 95.1%.
[0043]
Embodiment 2
[0045] Preparation of High Purity α-Type Intermediate Using Chloroform as Solvent
[0046] Under the protection of nitrogen, add 10g of pentaacetylglucose, 5.64g of hydroquinone, 10g of boron trifluoride ether and 50mL of chloroform into a 100mL reaction bottle, heat and reflux for 48h, the reaction solution is neutralized by sodium bicarbonate, and washed three times with saturated saline , dried over anhydrous magnesium sulfate, and concentrated to obtain 9.7 g of brown oil, wherein the content of α-isomer was 92.6%.
[0047]
Embodiment 3
[0049] Alternative reaction to Example 1
[0050] The mixture of the α / β-arbutin intermediate of 10g equimolar ratio, 50mL dichloromethane, 10g boron trifluoride ether drop into 100mL flask, operate with embodiment 1, reflux 48h, get brown oily matter 9.5g, Among them, the α-isomer content is 95.6%.
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com