Fluorescent probe for detecting biological hydrogen sulfide as well as preparation and application of fluorescent probe
A fluorescent probe, hydrogen sulfide technology, used in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of not being able to measure hydrogen sulfide in cells, large detection errors, etc., to achieve good biofilm permeability, avoid Interference, good effect of biofilm permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
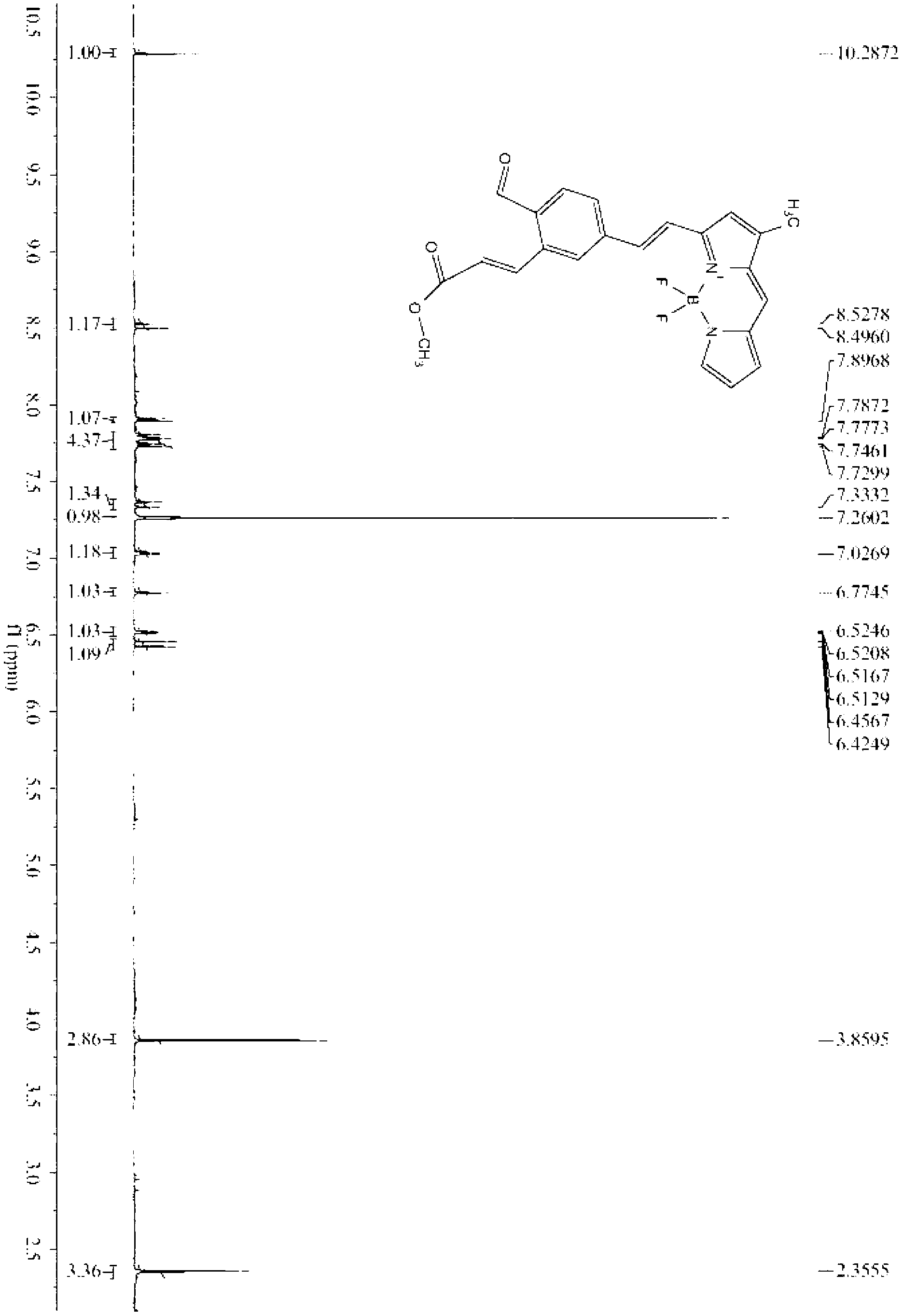
[0019] Embodiment 1: the preparation of fluorescent probe
[0020] The reaction formula is as follows:
[0021] .
[0022] The preparation steps are as follows:
[0023] (1) Preparation of formula II: appropriate amount of (E)-3-(2-((tert-butyldimethylsilyloxy)methyl)-5-formyl)phenyl-methyl acrylate (0.18-0.25 g ) and 1,3-dimethylfluoroborate dipyrrole fluorophore (0.10-0.15g) were dissolved in benzene (or toluene), added 0.1ml acetic acid and 0.1ml piperidine, heated to reflux for 4-5 hours, and divided The water tank removes the water generated by the reaction. After the reaction was completed, it was cooled to room temperature and quenched by adding water. The crude product was extracted with ethyl acetate, spin-dried, and purified by silica gel column chromatography.
[0024] (2) Preparation of formula III: the compound represented by formula II (0.10 g-0.20 g) was dissolved in acetonitrile, and 40% hydrofluoric acid aqueous solution (0.02-0.04 mL) was added dropwise...
Embodiment 2
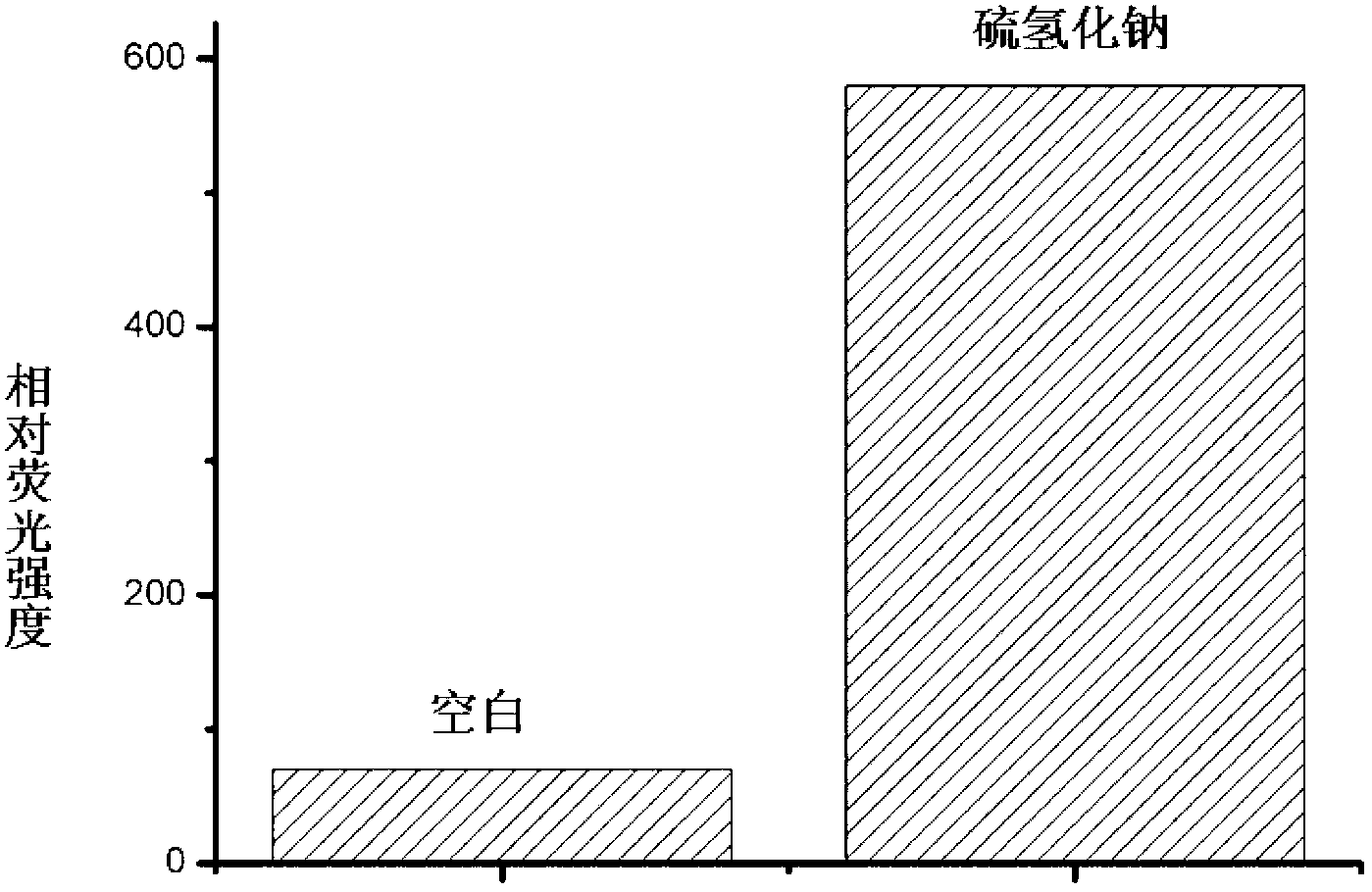
[0027] Example 2: Fluorescence changes before and after the reaction of probe molecules with hydrogen sulfide
[0028] Dissolve the probe molecule in a small amount of acetonitrile, add phosphate buffer solution or sodium hydrogensulfide phosphate buffer solution respectively, so that the final concentration of the probe molecule is 10 μM, and the final concentration of sodium hydrogensulfide is 100 μM. After 1 hour of reaction Measured on a fluorescence spectrometer, and then confirmed that the fluorescence intensity of the probe molecule was significantly enhanced after the reaction with hydrogen sulfide, such as figure 2 shown.
Embodiment 3
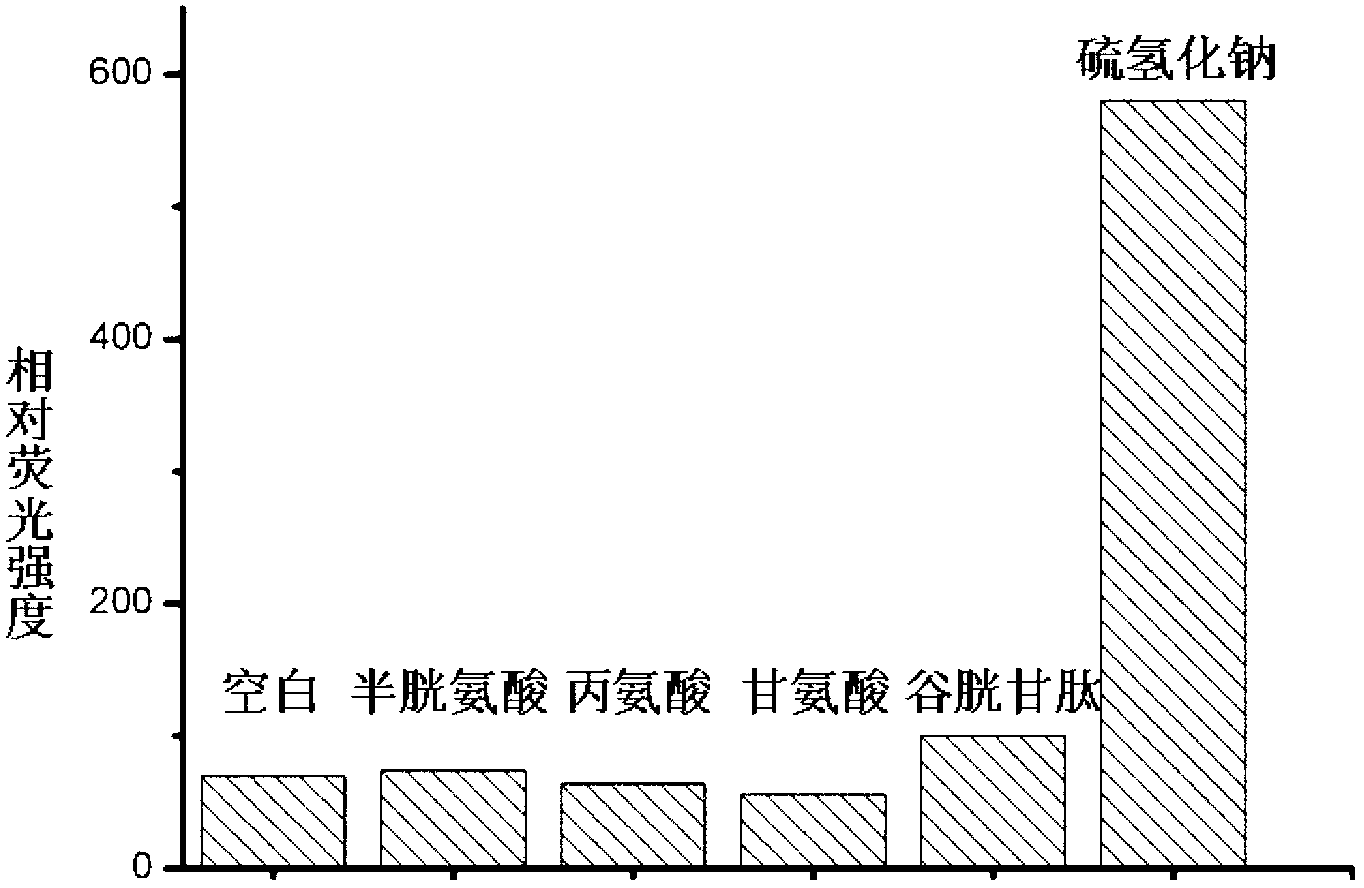
[0029] Embodiment 3: the selectivity of probe molecule to hydrogen sulfide
[0030] Dissolve the probe molecules with a small amount of acetonitrile, and then make a solution with phosphate buffer. The samples to be tested dissolved in phosphate buffer were added respectively so that the concentration of the final probe molecule was 10 μM and the concentration of the sample to be tested was 100 μM. After reacting for 1.0 hour, it was measured on a fluorescence spectrometer, and then the selectivity of the probe molecule to hydrogen sulfide was determined. Such as image 3 As shown, the probe molecule has high selectivity to hydrogen sulfide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com