Organic solar cell material containing triphenylamine-thiophene structure and its synthesis method
A technology of solar cells and triphenylamine, applied in the direction of organic chemistry, etc., can solve the problems of reducing the efficiency of charge separation, low efficiency of Schottky cells, etc., and achieve the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
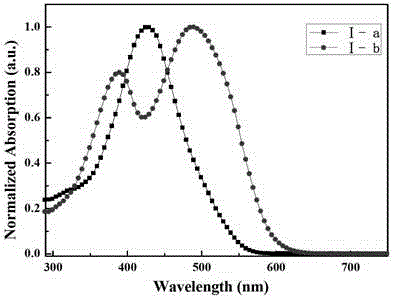
[0085] What this embodiment synthesizes is the organic solar cell material containing triphenylamine-thiophene structure, and its molecular formula is C 154 h 182 N 4 o 4 P 4 Pt 2 S 12 , whose structural formula is (I–a):
[0086]
[0087] Its synthetic method comprises the following steps:
[0088] Step 1: Obtain R1 according to the synthesis method of the bridging unit R1 of the above-mentioned π-conjugated structure;
[0089] Step 2: Obtain G according to the specific synthesis route of the above-mentioned organometallic aryne ligand containing the triphenylamine-thiophene structure;
[0090] Step 3: Weigh 0.15g (0.21mmol) R1 into a 100mL three-necked flask, add 50mL of a mixed solution of dichloromethane and triethylamine (V:V=1:1), and add 10% mol of iodide under nitrogen atmosphere Cuprous, then added 0.64g (0.53mmol) of G, stirred at room temperature and maintained the reaction for 2 hours. Concentrated and separated by thin-layer chromatography to obtain 0....
Embodiment 2
[0092] What this embodiment synthesizes is the organic solar cell material containing triphenylamine-thiophene structure, and its molecular formula is C 166 h 182 N 12 P4 Pt 2 S 12 , whose structural formula is (I–b):
[0093]
[0094] Its synthesis method is the same as that of Example 1, except that in step 3, add 0.20 g (0.066 mmol) of the obtained aldehyde-containing battery material I-a into a 100 mL one-necked bottle, add 40 mL of dichloromethane to completely dissolve it, and then add 35 mg (0.53 mmol) of malononitrile and 5 drops of triethylamine were stirred at room temperature for reaction, detected by TLC until the end of the reaction; concentrated, chromatographed with methanol and washed several times to finally obtain 0.20 g of dark red solid with a yield of 94.1% , according to NMR analysis, the product is a cyano-containing battery material I–b: 1 HNMR (400MHz, CDCl 3 ):δ(ppm)7.78(s,4H),7.70(d,J=3.2Hz,4H),7.61(d,J=8.4Hz,8H),7.51(d,J=8.0Hz,4H),7.38 (d,...
Embodiment 3
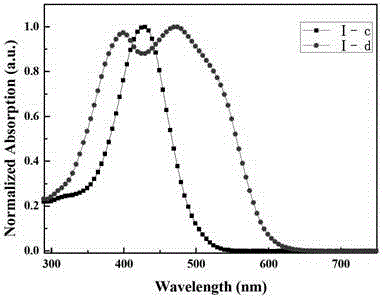
[0096] What this embodiment synthesizes is the organic solar cell material containing triphenylamine-thiophene structure, and its molecular formula is C 156 h 190 N 2 o 4 P 4 Pt 2 S 11 , whose structural formula is (I–c):
[0097]
[0098] Its synthesis method is the same as that of Example 1, the only difference being that the π-conjugated Overseas Chinese Union unit with R2 structure is used in Step 1.
[0099] NMR analysis data: 1 HNMR (400MHz, CDCl 3 ):δ(ppm)9.88(s,4H),7.73(d,J=3.6Hz,4H),7.58(d,J=8.4Hz,8H),7.49(d,J=8.4Hz,4H),7.34 (d,J=3.6Hz,4H),7.17(d,J=8.4Hz,8H),7.13(d,J=8.0Hz,4H),7.07(d,J=3.6Hz,2H),7.03(d ,J=3.6Hz,2H),7.00(d,J=3.6Hz,2H),6.96(d,J=3.2Hz,2H),6.81(d,J=3.6Hz,2H),6.74(d,J =3.6Hz,2H),2.70(m,4H),2.12-2.11(m,24H),1.60-1.59(m,24H),1.53-1.44(m,24H),1.28(m,16H),0.95( t,J=7.2Hz,36H),0.88(m,6H); 13 CNMR (100MHz, CDCl 3 ):δ(ppm)182.67,153.99,147.96,144.98,141.74,140.17,139.90,137.66,134.31,133.63,131.07,129.76,128.44,128.04,127.60,127.48,126.54,126.24,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com