Preparation method of hydrogenated lithium titanate nano-material
A technology of lithium hydride titanate and nanomaterials, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., can solve the problems of small particle size, high preparation cost, and poor conductivity of lithium hydride titanate, and achieve huge commercial potential, lower calcination temperature, and improve lithium storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
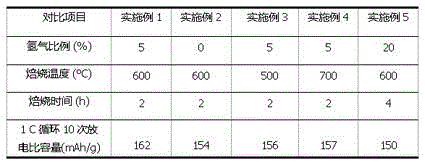
[0026] (1) Add 0.4 g (0.05 mol) of titanium dioxide powder to 40 mL of 2.5 M LiOH (0.1 mol) aqueous solution, stir well, and then place it in an oven at 80 °C for 10 h in a closed environment;
[0027] (2) Stop heating, naturally cool the sample in step 1 to room temperature, suction filter, wash to neutrality, and dry to obtain lithium titanium oxide precursor powder;
[0028] (3) The precursor powder in step 2 is calcined at 600 °C for 2 h in a mixed atmosphere of hydrogen and argon containing 5% (volume percent) of hydrogen to obtain lithium hydride titanate nanomaterials.
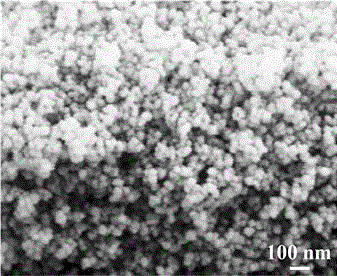
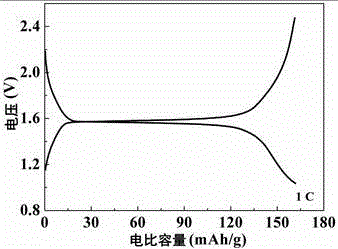
[0029] Electron microscope scanning is performed on the obtained lithium hydride titanate nanomaterials, such as figure 1 It can be seen that the obtained lithium titanate material is nanoparticles with good dispersibility, the particle size distribution is 15-30 nm, and the electronic conductivity is 3.8×10 -7 S cm -1 , the specific capacity curve of the material by assembling into a button battery ...
Embodiment 2
[0031] (1) Weigh 0.4 g (0.05 mol) of titanium dioxide powder into 40 mL of 2.5 M LiOH (0.1 mol) aqueous solution, stir evenly, and then place it in an airtight oven at 80°C for 10 h;
[0032] (2) Stop heating and cool the sample in step 1 to room temperature naturally. Take out the sample for suction filtration, wash to neutrality, and dry to obtain lithium titanium oxide precursor powder;
[0033] (3) calcining the precursor powder in step 2 at 600 °C for 2 h in an air atmosphere to obtain lithium titanate nanomaterials .
[0034] The obtained lithium hydride titanate nanomaterial was tested, and the electronic conductivity was measured to be 2.1×10 -12 S cm -1 .
Embodiment 3
[0036] (1) Weigh 0.4 g (0.05 mol) of titanium dioxide powder into 40 mL of 2.5 M LiOH (0.1 mol) aqueous solution, stir evenly, and then place it in an airtight oven at 80°C for 10 h;
[0037] (2) Stop heating and cool the sample in step 1 to room temperature naturally. Take out the sample for suction filtration, wash to neutrality, and dry to obtain lithium titanium oxide precursor powder;
[0038] (3) The precursor powder in step 2 was calcined at 500 °C for 2 h in a mixed atmosphere of 5% hydrogen and argon to obtain lithium hydride titanate nanomaterials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com