Methadone hapten, preparation method of methadone hapten, methadone antigen, methadone monoclonal antibody and application of methadone monoclonal antibody
A monoclonal antibody and methadone technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxyl compounds, etc., can solve the problems of time-consuming and cumbersome operations, and achieve simple preparation methods, easy-to-obtain raw materials, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Preparation of Methadone Monoclonal Antibody
[0066] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, a complete antigen of the invention can be administered to an animal to induce the production of monoclonal antibodies. Monoclonal antibodies can be produced using hybridoma technology or can be produced using recombinant DNA methods.
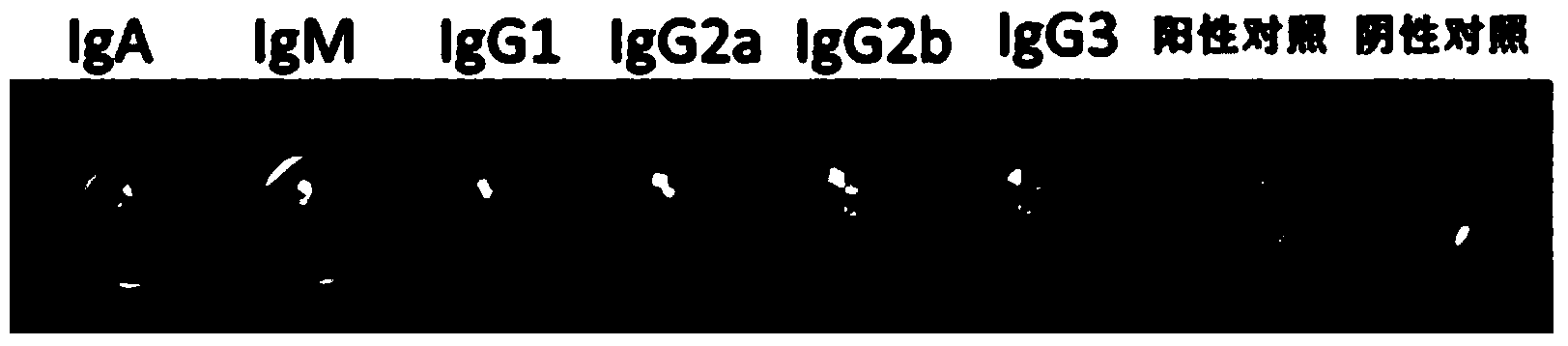
[0067] The invention provides an anti-methadone monoclonal antibody MTD-5, which is identified as IgG1 type. In a preferred solution of the present invention, the monoclonal antibody MTD-5 is prepared by culturing hybridoma cells. The supernatant of the hybridoma cell culture was taken, the IgG was crudely extracted by saturated ammonium sulfate precipitation, and then the crudely extracted antibody was purified by an affinity chromatography column.
[0068] In a preferred embodiment of the present invention, the monoclonal antibody MTD-5 is prepared by a method...
Embodiment 1
[0102] Example 1 Preparation of methadone hapten
[0103] A methadone hapten, the methadone hapten is prepared by the following method, the specific steps are as follows:
[0104] (1) To reduce methadone to hydroxymethadone, the specific steps are: put methadone hydrochloride (2.4mmol) in a 50ml round bottom flask, add 10ml of anhydrous methanol and stir to dissolve, add sodium borohydride (4.8mmol) under ice bath, The temperature was controlled at 5°C for reaction. After completion of the reaction, add 30ml of ice water and stir to dissolve, extract 3 times with 15ml of ethyl acetate, combine the organic phases, wash twice with saturated aqueous sodium chloride solution, collect the organic phases, and dry over anhydrous sodium sulfate for 1 hour. The mother liquor was collected by filtration, and the solvent was evaporated to dryness.
[0105] (2) Dissolve the hydroxymethadone obtained in step (1) in 5ml of toluene, add succinic anhydride, and react at 110°C for 20 hours. ...
Embodiment 2
[0106]Example 2 Preparation of methadone-OVA complete antigen
[0107] A kind of methadone-OVA complete antigen, described methadone-OVA complete antigen is to use carbodiimide method (EDC) method to make methadone hapten and protein carrier connection, concrete steps are as follows:
[0108] Dissolve 9.0mg (0.029mmol) of methadone hapten in 500μl N,N-dimethylformamide, shake and mix well, add EDC0.04mmol, NHS0.04mmol and stir at 4°C for 4 hours;
[0109] Dissolve the carrier protein OVA0.003mmol in 2.0ml PBS (0.05M, pH7.0), slowly add to the above solution, after the addition, stir and react at room temperature for 24 hours, then dialyze in distilled water for 2 days, change the solution 3 times, Centrifuge in a high-speed refrigerated centrifuge, collect the supernatant, and obtain the methadone-OVA immunogen.
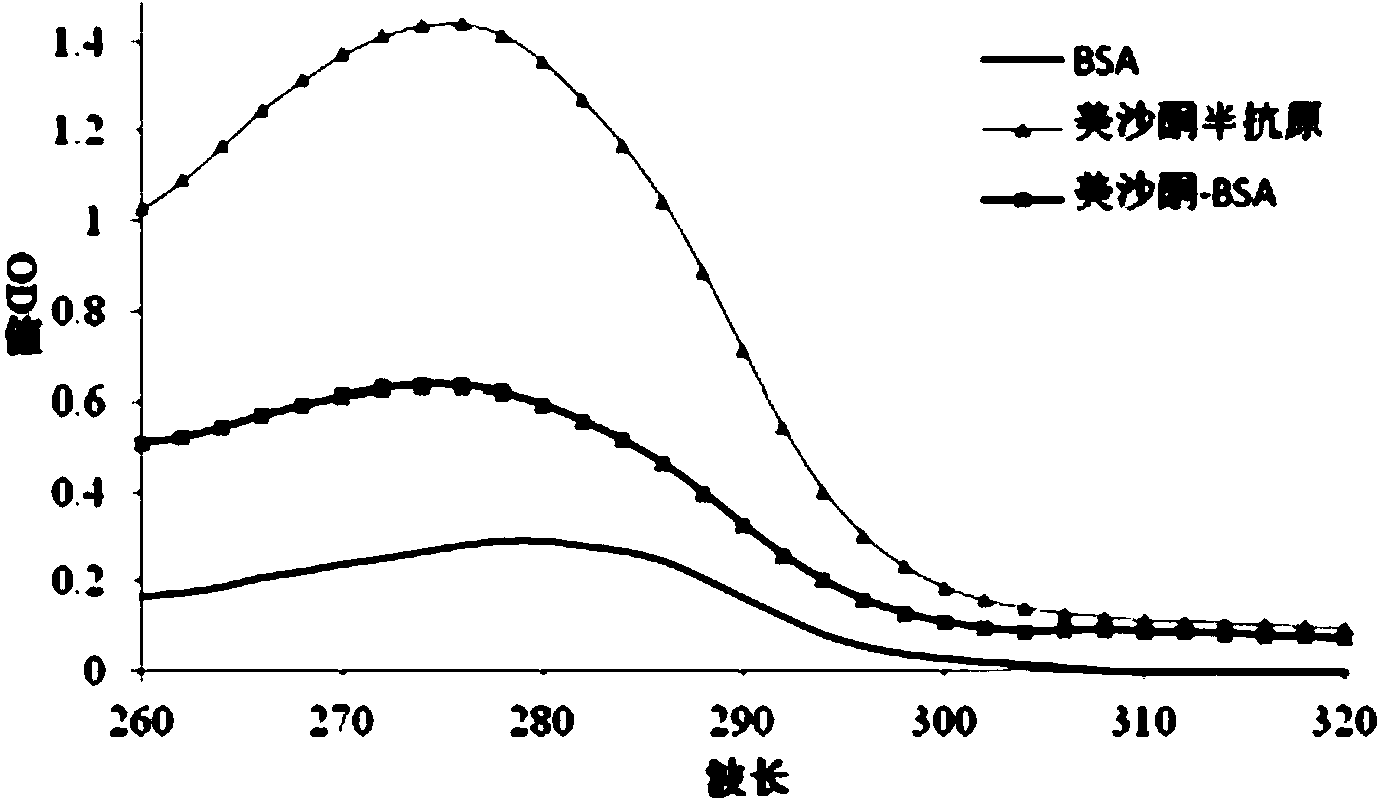
[0110] Visible by UV scanning (see figure 2 ), the maximum absorption peaks of the three in the range of 260-310nm are obviously different, that is, the coupling ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com