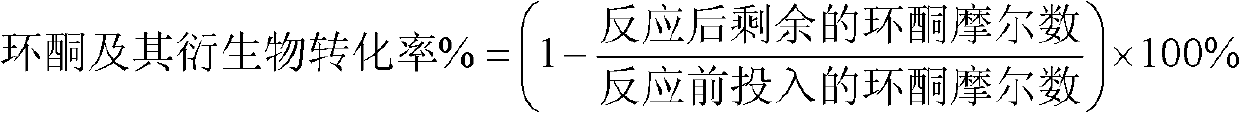Reaction method for preparing corresponding lactone, hydroxy acid and dicarboxylic acid through cyclic ketone oxidation
A technology of dicarboxylic acid and hydroxy acid, which is applied in the preparation of carboxylic acid by oxidation, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor atom economy, low selectivity, easy to fly temperature, etc. The effect of stable control, increasing hydrogen peroxide concentration and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example illustrates the direct catalytic oxidation of cyclohexanone to produce ε-caprolactone, 6-hydroxycaproic acid and adipic acid.
[0037] The reactor is a slurry bed reactor, the catalyst used is nano-beta molecular sieve, and the particle diameter distribution of the catalyst is 1-200 μm; the reaction raw materials are 30% hydrogen peroxide and more than 99.9% cyclohexanone; the entrainer is ethyl acetate. Prepare the ethyl acetate slurry with a catalyst concentration of 5% in the catalyst storage tank, and fully stir and disperse it for later use. Use ethyl acetate to test run the device under the set conditions. After the device is stable, mix the catalyst slurry, hydrogen peroxide The raw material cyclohexanone is added to the reaction section in the tower. The absolute pressure is 0.1MPa, the reaction setting temperature is 75°C, the azeotropic temperature in the reactor is 74°C, the molar ratio of cyclohexanone to ethyl acetate in the reaction section is...
Embodiment 2
[0039] This example illustrates the direct catalytic oxidation of cyclohexanone to produce ε-caprolactone, 6-hydroxycaproic acid and adipic acid.
[0040] Weigh 20g of nano-beta molecular sieve and put it into 50ml of dilute sulfuric acid solution with a concentration of 25%, stir continuously at 70°C for 2 hours, filter, dry and roast in a muffle furnace at 550°C for 3 hours, and take it out to get an alumina content of 2.3 wt% of acid-eluted aluminum nano-beta molecular sieves. Weigh 1.36g of zinc chloride solid and fully dissolve it in 10ml of water, add 10g of the prepared acid-eluted aluminum nano-beta molecular sieve and impregnate it in equal volume at room temperature for 6 hours, then put it in an oven at 110°C for 3 hours, and then place it at 550 ℃ in a muffle furnace for 3 hours, and the ZnO / acid-eluted aluminum nano-beta molecular sieve with a zinc oxide content of 8.14 wt% was obtained after taking it out.
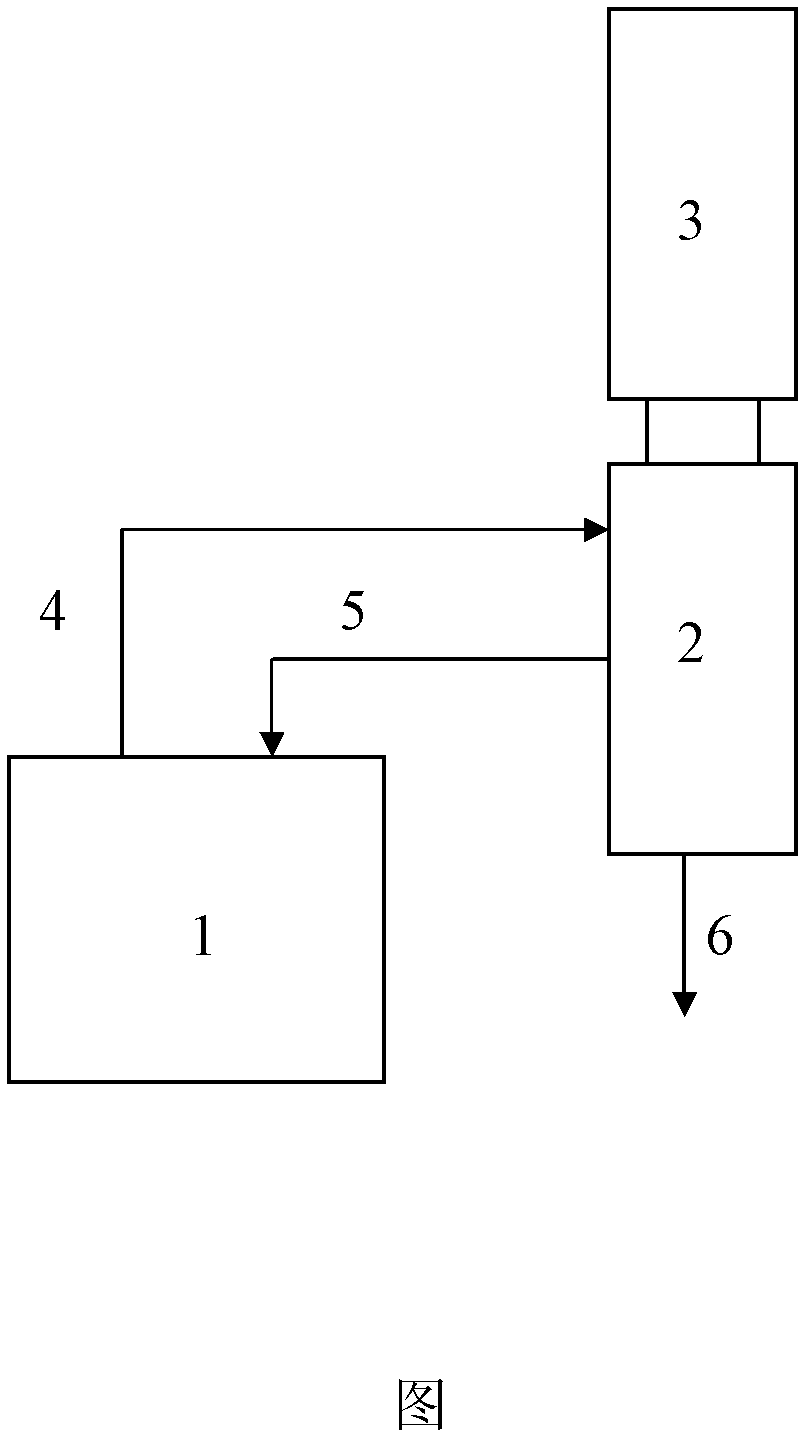
[0041]The test was operated according to the diagram, ...
Embodiment 3
[0043] This example illustrates the catalytic oxidation of cyclopentanone to δ-valerolactone, 5-hydroxyvaleric acid and glutaric acid. Weigh 30g of nano-beta molecular sieve and put it into 50ml of HCl solution with a concentration of 15%, stir continuously at 90°C for 2 hours, filter, dry and roast in a muffle furnace at 550°C for 3 hours, and take it out to get an alumina content of 2.9wt % of acid-eluted aluminum nano-beta molecular sieves. Weigh 3.55g of tin tetrachloride pentahydrate solid and fully dissolve it in 15ml of water, add 20g of the prepared acid-eluted aluminum nano-beta molecular sieve, impregnate in equal volume at room temperature for 6 hours, and then put it in an oven at 110°C for 3 hours. Then bake in a muffle furnace at 550°C for 3 hours, and take it out to obtain SnO with a zinc oxide content of 7.51wt%. 2 / Acid-eluted aluminum nano-beta molecular sieve.
[0044] The test was operated according to Figure 1, the reactor was a slurry bed reactor, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com