Polyesteramide and polyethyleneglycol periodic copolymer and preparation method thereof
A block copolymer and polyethylene glycol technology, applied in the field of biomedical polymer materials, can solve the problems of high cardiotoxicity and myelosuppressive toxicity, endangering the lives of patients, etc., to reduce toxic and side effects, simple steps, and expand the scope of application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
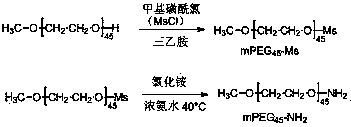
[0028] Example 1: Synthesis of polyethylene glycol with terminal amino groups.
[0029]Polyethylene glycol monomethyl ether (mPEG) with a molecular weight of 2000 45 -OH) The terminal hydroxyl group is modified into an amino group. The specific synthesis method is:
[0030]
[0031] Take 30 g mPEG 45 Add 300 mL of toluene to -OH to azeotropically remove water, distill off excess toluene after 4 hours, add dry 200 mL of dichloromethane and 10.50 mL of triethylamine to the reaction flask, add 4.65 mL of mL of methanesulfonyl chloride in 25 mL of dichloromethane solution, after 24 hours of reaction, remove the triethylamine hydrochloride produced by filtration, wash the reaction solution three times with NaCl solution, separate the layers, and wash with anhydrous NaSO 4 The solution was dried, filtered, concentrated by rotary evaporation, and precipitated with ether to obtain polyethylene glycol monomethyl ether methanesulfonate (mPEG 45 -Ms), dried under vacuum at 40 °C t...
Embodiment 2
[0035] Example 2: p-nitrophenol sebacate (electrophilic monomer) and 1,6-hexanediol diphenylalanine ester p-toluenesulfonate (nucleophilic monomer) synthesis.
[0036] Sebacic acid p-nitrophenol ester (electrophilic monomer) is synthesized according to the synthetic route shown below. The specific synthesis method is:
[0037]
[0038] Dissolve 43.00 g of p-nitrophenol and 43.13 mL of triethylamine in 500 mL of acetone, and cool the solution in an ice-water bath at 0 °C. Dissolve 28.54 mL of sebacoyl chloride in 100 mL of acetone and drop into the p-nitrophenol solution. The reaction is gradually warmed to room temperature and carried out under stirring for 12 hours. After the reaction, pour into 2000 mL of distilled water to precipitate the product, filter it with suction, recrystallize three times with ethyl acetate, and dry it under vacuum at 40°C to constant weight, with a yield of 75%.
[0039] 1,6-Hexanediol diphenylalanine ester p-toluenesulfonate (nucleophilic mo...
Embodiment 3
[0042] Example 3: Polyesteramide and mPEG 45 -NH 2 Amphiphilic triblock copolymer (mPEG 45 -PEA-mPEG 4 )Synthesis.
[0043] Through solution polycondensation, the molecular weight of the polyester amide chain segment is controlled by changing the ratio of nucleophilic and electrophilic monomers. The polyester amide segment molecular weight synthesized in the present embodiment is 2000, and concrete synthetic method is:
[0044] Under the protection of nitrogen, take 0.50 g of 1,6-hexanediol diphenylalanine ester p-toluenesulfonate, 0.37 g of p-nitrophenol sebacate (the molar ratio of nucleophilic and electrophilic monomer substances is 4 : 5) Add 0.20 mL triethylamine and 0.13 mL dimethylacetamide to a 10 mL reaction flask, heat up to 80°C under stirring conditions, and react for 12 hours, then add 0.66 g mPEG 45 -NH 2 After adding the reaction flask to continue the reaction for 12 hours, the reaction temperature was lowered to room temperature, and 4 mL of dimethylforma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com