Furanone-aryl-oxazolidinone type compound as well as preparation method and application thereof
A technology of oxazolidinone and furanone, which is applied in the application field of preparing antibacterial drugs, can solve the problems that there are no dual-target antibacterial compounds, and achieve good inhibition and killing effects and high antibacterial activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
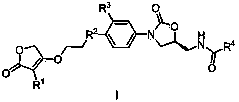
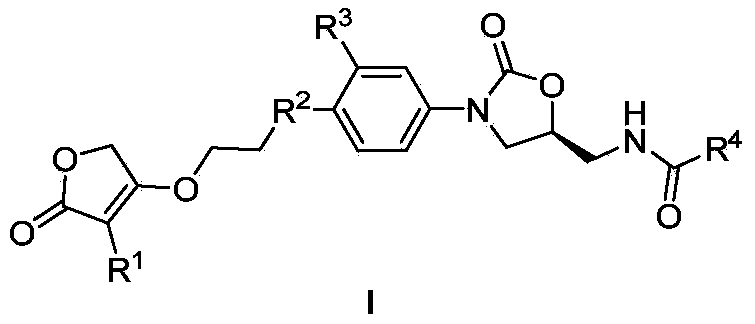
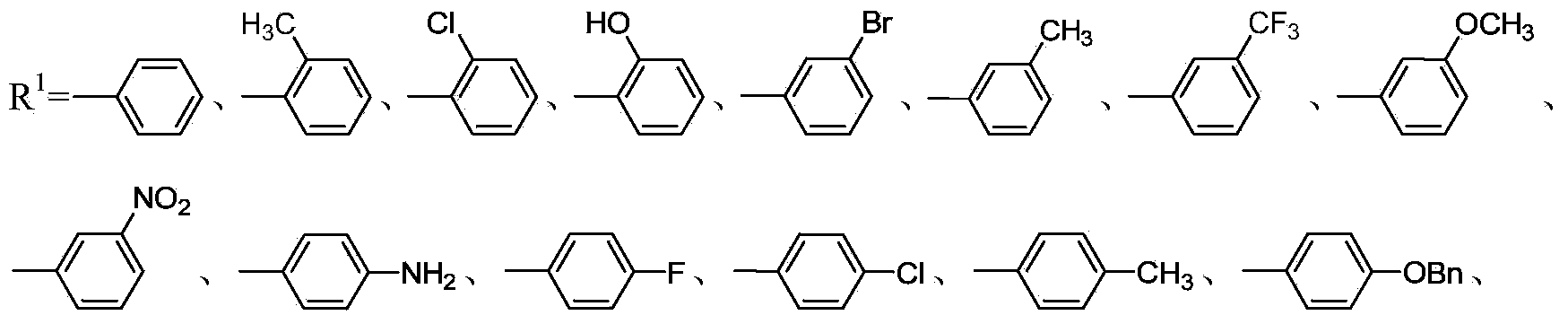
[0026] Example 1: (S)-3-(4-(4-(3-(3-methoxyphenyl)-2(5H)-furanone-4-yloxyethyl)piperazin-1-yl )-3-hydroxyphenyl)-5-benzamidomethyl-2-oxazolidinone (29)
[0027] Step 1: Dissolve 1.88g (10mmol) sodium 3-methoxyphenylacetate in 15mL DMSO, add 1.2mL ethyl bromoacetate at room temperature, raise the temperature to 35°C and react for 8h, after the reaction is complete, dilute with ethyl acetate and wash with water , the organic layer was washed with saturated brine until neutral, dried, concentrated, and subjected to silica gel column chromatography, the eluent was petroleum ether-AcOEt, and the volume ratio of petroleum ether to AcOEt was 4:1 to obtain 2.2g oily 2-(3 -ethyl methoxyphenylacetoxy)acetate;
[0028] Step 2: Dissolve 2.01 g of ethyl 2-(3-methoxyphenylacetoxy) acetate in a constant pressure funnel containing 10 mL of anhydrous THF, and add 0.19 g of NaH into a flask containing 5 mL of anhydrous THF 2-(3-methoxyphenylacetoxy) ethyl acetate solution in THF was slowly ad...
Embodiment 2
[0042] Embodiment 2: the antibacterial activity of compound
[0043] Bacteria were suspended in MH medium at a concentration of approximately 10 5 cfu. mL -1 , add the bacterial solution to a 96-well plate (100 μL of bacterial solution per well), use the culture medium as a blank control, use DMSO instead of a test substance as a negative control, use penicillin G as a positive control for Gram-positive bacteria, and use penicillin G as a positive control for Gram-positive bacteria. Kanamycin was used as a positive control for Shi-negative bacteria, and ketoconazole was used as a positive control for fungi. Dissolve the test substance in DMSO to prepare 1600, 800, 400, 200, 100, 50 μg. mL -1 solution (for MIC 50 Less than 5μg. mL -1 Yes, when carrying out one-step experiment, the prepared concentration gradient is 100, 50, 25, 12.5, 6.25μg. mL -1 ), was added to a 96-well plate at an amount of 11 μL per well, and four parallel experiments were performed for each conce...
Embodiment 3
[0046] Example 3: Activity of ribosomal protein synthesis
[0047] Take the Escherichia coli liquid in the logarithmic growth phase, centrifuge and wash the cells twice with 5mL buffer solution at 3°C. The composition of the buffer solution is as follows: 0.01M Tris (pH7.8), 0.017M magnesium acetate and 0.06M potassium chloride. The resulting cells were frozen at -70°C, and after thawing, they were ground for 15 minutes with aluminum oxide twice the wet weight of the cells to obtain a crude extract of S30 ribosomes. Dissolve the crude extract of S30 ribosomes in 0.25mL of 0.017M magnesium acetate buffer, add a certain concentration of the test compound, incubate at room temperature for 15min, and then add primer polyuridine, 4×10 -9 mol[ 14 C] phenylalanine, 5×10 -9 mol of phenylalanine and 5 x 10 -9 mol of other essential amino acids, continue to incubate for 15 min. The synthesized protein was precipitated by adding 1 mL of 10% trichloroacetic acid solution at 3 °C, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com