Preparation method of compound effervescent tablets for treating chicken infectious bursal disease
A technology for infectious and bursal disease of chickens, which is applied in the directions of medical preparations, pharmaceutical formulations, antiviral agents, etc. containing active ingredients, which can solve the inconvenience of storage and transportation for users, it is difficult to ensure accurate measurement of animals, and reduce the risk of living. The number of bacteria and other issues can improve the overall immune effect, enhance the immunity of chickens, and reduce stress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
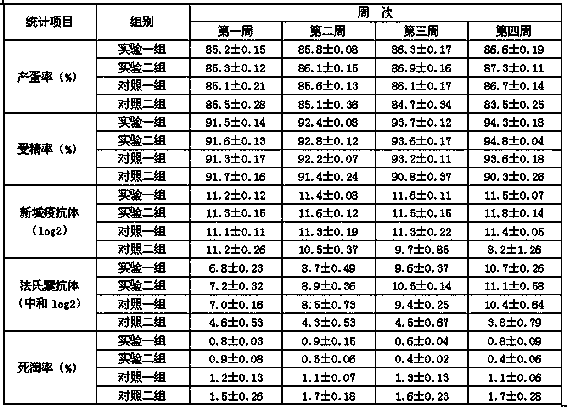
Image
Examples
Embodiment 1
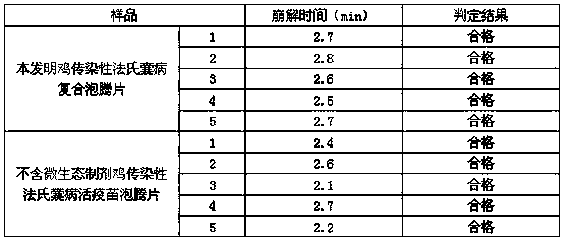
[0036] Embodiment 1: use DF-1 cell line to cultivate chicken infectious bursal virus to prepare chicken infectious bursal virus suspension
[0037] (1) Passage and culture of cells for seedling production: take chicken embryo fibroblast passage cell line DF-1 cell line, digest and passage with trypsin cell dispersion liquid, and continue to culture with cell growth liquid. When a good monolayer is formed, it is used to continue passaging or inoculation with virus;
[0038] (2) Propagation of cytotoxic seeds: Take the infectious bursal virus B87 strain (purchased from the China Veterinary Drug Administration) virus seeds for production, and inoculate 1% of the maintenance fluid volume in chickens that have grown into a good monolayer. Place embryonic fibroblast passage cells in the maintenance medium at 37-38°C to continue culturing, and harvest the cell fluid when the cell lesion rate reaches 75% or more;
[0039] (3) Propagation of venom for seedling production: take the abo...
Embodiment 2
[0041] Embodiment 2: the preparation of probiotic mixed bacteria liquid
[0042] (1) Cultivation of strains
[0043] Culture of lactic acid bacteria
[0044] Preheat the MRS liquid medium to 37°C, and inoculate the lactic acid bacteria seed liquid at 2% inoculum, let it stand at 37°C for 18-24 hours, harvest the bacterial liquid and store it at -4°C for later use;
[0045] Culture of Bacillus
[0046] Preheat the Bacillus culture medium to 37°C, at the same time inoculate the Bacillus seed solution with 1% inoculation amount, let it stand at 37°C for 18-24 hours, harvest the bacteria solution and store it at 4°C for later use;
[0047] Described lactic acid bacteria are: Bifidobacterium bifidum ( Bifidobacterium bifidum ), Lactobacillus ( Lactobacillus lactis ), Lactobacillus Brucella ( Lactobacillus buchneri ), Bifidobacterium longum ( Bifidobacterium longum ) in one or more;
[0048] Described bacillus is: Bacillus licheniformis ( Bacillus licheniformis ), Bacill...
Embodiment 3
[0055] Embodiment 3: the preparation of Chinese medicine polysaccharide and Chinese medicine flavone
[0056] a. Codonopsis 15 parts, ginseng 18 parts, wolfberry 13 parts, Andrographis paniculata 20 parts, Achyranthes bidentata 15 parts, Poria cocos 20 parts, Ginkgo biloba 30 parts, licorice 20 parts, astragalus 20 parts, nettle 15 parts, quince 20 parts 15 parts of Salvia miltiorrhiza, weighed each raw material of Chinese medicine, chopped, washed, soaked in cold water overnight, then added purified water 15 times the weight of the raw material, water bathed to 90 ° C, and kept at 90 ° C, boiled for 2 hours, boiled Stir every 10 minutes during the process;
[0057] b. Discard the dregs of the traditional Chinese medicine in a, collect the medicinal liquid, and centrifuge at 10,000 rpm for 10 minutes after cooling at room temperature, discard the precipitate, and collect the supernatant;
[0058] c. The supernatant in b is subjected to alcohol precipitation, the precipitation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com