Enzymatic preparation of naloxone and pharmaceutical composition thereof
A technology of enzymatic preparation and naloxone, which is applied in the field of medicine, can solve the problems of reagents that are harmful to the human body, severe conditions, and not jumping out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
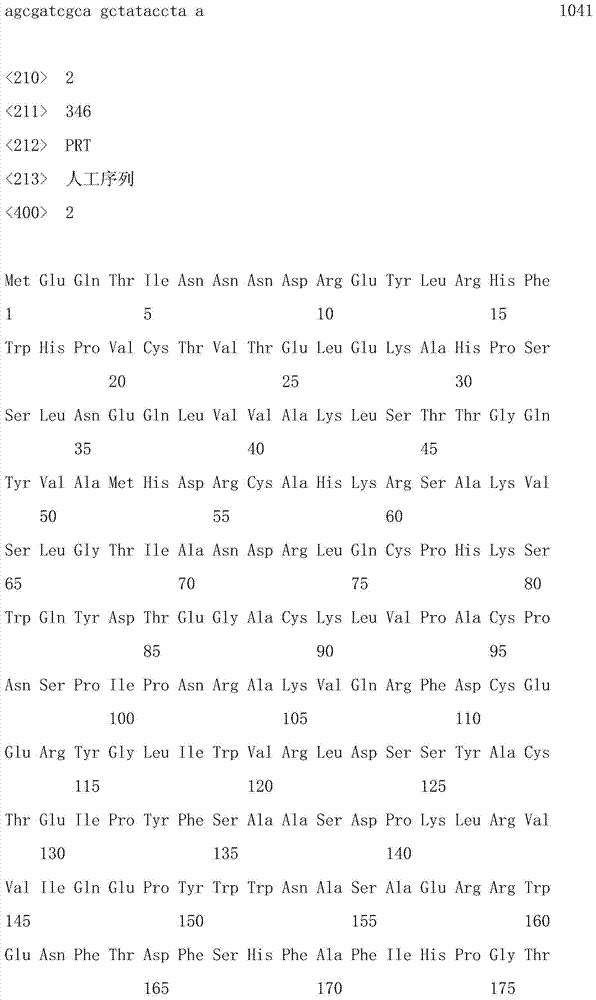
[0041] The expression of embodiment 1 demethylase gene
[0042] A new enzyme gene was designed according to our platform technology, and Sangon Bioengineering (Shanghai) Co., Ltd. was commissioned to synthesize a demethylase gene optimized for codon expression. Its nucleotide sequence is shown in SEQ ID No: 1 in the sequence table , the amino acid sequence encoded by the gene is shown in SEQ ID No:2. Then, construct the yeast that secretes and expresses the enzyme according to the "Molecular Cloning Experiment Guide", that is, use the synthetic gene as a template, use primer 1 (SEQ ID No: 3, introduces the EcoR I endonuclease site) and primer 2 (SEQ ID No:4, the Not I endonuclease site was introduced) After PCR amplification, EcoR I and Not I were double-digested, and the pPIC3.5 plasmid (available from Invitrogen Corporation) digested with these two endonucleases ) were ligated with T4 DNA ligase and transformed into Escherichia coli DH5α. Pick out the positive clones and e...
Embodiment 2
[0044] The production of embodiment 2 naloxone
[0045] (1) Chemical synthesis of 14-hydroxy-7,8-dihydrocodone
[0046] Basically according to the existing technology of hydrogen oxidation synthesis, the specific improvement is as follows: Weigh 50g of thebaine and add it to 100mL of anhydrous formic acid in an ice bath, stir and dissolve, and add 30% (V / V) peroxide under ice bath conditions 22 mL of hydrogen solution, then stirred for 1 hour, then warmed up to room temperature 37°C and continued to stir for 2.5 hours. Then, add 60mL of acetone and 60mL of water to the reaction solution, then add dropwise 20% (W / W) sodium hydroxide solution until the pH reaches 8.2, and stand still for 0.5 hours after the dropwise addition, during which precipitation will precipitate, filter and dry to obtain a white powder (14-hydroxycodeinone) 44.3g. The above white powder was dissolved in 200mL of 10% (W / W) acetic acid solution, 4g of Pd / C catalyst was added, and hydrogen gas was introduc...
Embodiment 3
[0051] Drug efficacy and safety test of embodiment 3 naloxone composition
[0052] Experimental drug: the naloxone composition prepared in Example 2 was directly administered after being neutralized with hydrochloric acid without high-cost purification.
[0053] Control drug: naloxone hydrochloride injection (Yiqiao (Hunan) Pharmaceutical Co., Ltd.).
[0054]Experimental method: BALB / c mice (body weight 18-22g, half male and half female) were randomly divided into the following 6 groups, with 10 animals in each group: 1) blank control group (administered with distilled water); 2) model group (administered with distilled water) 50% ethanol solution 1mL); 3) high-dose control group (injection 0.5 mg / kg naloxone + 1 mL 50% ethanol solution for intragastric administration); 4) low-dose control group (injection 0.1 mg / kg·day as naloxone + 1 mL of 50% ethanol solution for intragastric administration); 5) high-dose experimental drug group (administered with naloxone and The total a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com