Nucleic acid detection method based on polymer electrochemiluminescence signal amplification technology
A technology of luminescent signal and detection method, which is applied in the direction of biochemical equipment and methods, measurement/inspection of microorganisms, etc., can solve the problems of not achieving ideal amplification effect, unstable labeling process, complicated reaction process, etc., and achieves convenience for modification and storage , easy to save, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
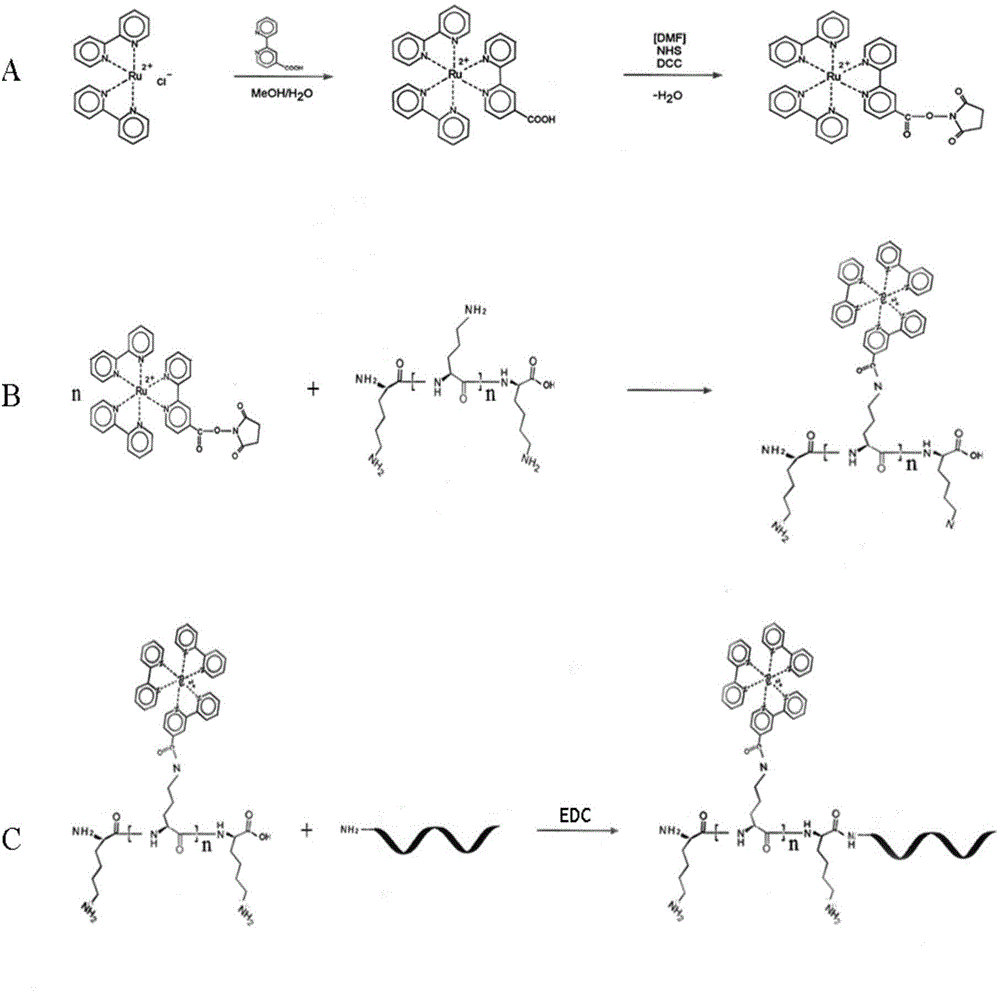
[0063] Synthesis and activation of embodiment 1 terpyridine ruthenium
[0064] (1) The synthesis process of terpyridine ruthenium is as follows:
[0065] ① Electronic balance weighs: dichlorobis(2,2'-bipyridine) ruthenium (Ru(bpy) 2 Cl 2 ) (CAS: 15746-57-3) 0.05g, NaHCO 3 0.05g, 0.0375g of 4-carboxylic acid-2,2'-bipyridine in a 50mL round bottom flask.
[0066] ② Add 10 mL of 80% methanol aqueous solution (MeOH), and heat the mixture under reflux in a silicone oil bath at 80°C for 10 h.
[0067] ③ The resulting solution was cooled in an ice bath (ice-water mixture) for 2 hours.
[0068] ④ Use 1mol / L of H 2 SO 4 Adjust the pH to 4.4 (calibrated with precision pH paper), and a precipitate forms.
[0069] ⑤ Filter the formed precipitate with filter paper and collect the filtrate.
[0070] ⑥Add 3.125mL NaPF to the filtrate obtained in ⑤ 6 (CAS: 21324-39-0) solution (2.5gNaPF 6 dissolved in 12.5mL of water), stirred and placed in the refrigerator at 4°C to evaporate the f...
Embodiment 2
[0079] The preparation of embodiment 2 polylysine-terpyridine ruthenium complex:
[0080] ① Take a tube of polylysine solid powder (5mg) and centrifuge at 12000r / min for 5min, so that the polylysine solid powder stuck on the tube wall falls to the bottom of the tube.
[0081] ② Gently open the cap of the tube, and quickly add 75 μL of 0.1M sodium borate (adjust the pH value to 8.5 with dilute hydrochloric acid), and immediately cap the tube.
[0082] ③ Fully oscillate up and down for 5 minutes, then centrifuge at 12000r / min for 5 minutes to collect, so that the liquid sticking to the tube wall falls to the bottom of the tube.
[0083] ④ Add 30 μL of the activated ruthenium terpyridine obtained in Example 1.
[0084] ⑤ Wrap it with black tape, place it on a centrifuge tube rack and incubate at room temperature in the dark for 12 hours.
[0085] ⑥Use an ultrafiltration tube (100KDa) to separate and remove small molecule compounds in the system.
[0086] ⑦ Centrifuge at 12000r...
Embodiment 3
[0095] Example 3 Magnetic capture detection target DNA sequence:
[0096] In this part, based on the traditional magnetic capture and "sandwich" model, a nucleic acid detection method using polylysine-terpyridine ruthenium as the signal amplification group is constructed.
[0097] (I) Dissolve the biotin probe and target sequence in water with a final concentration of 10 μM.
[0098] (II) Construct a magnetic capture nucleic acid detection system: the total volume of the system is 100 μL, the final concentration of biotin probe and polylysine is 100 nM, the final concentration of PBS buffer is 1×, add water to make up to 100 μL.
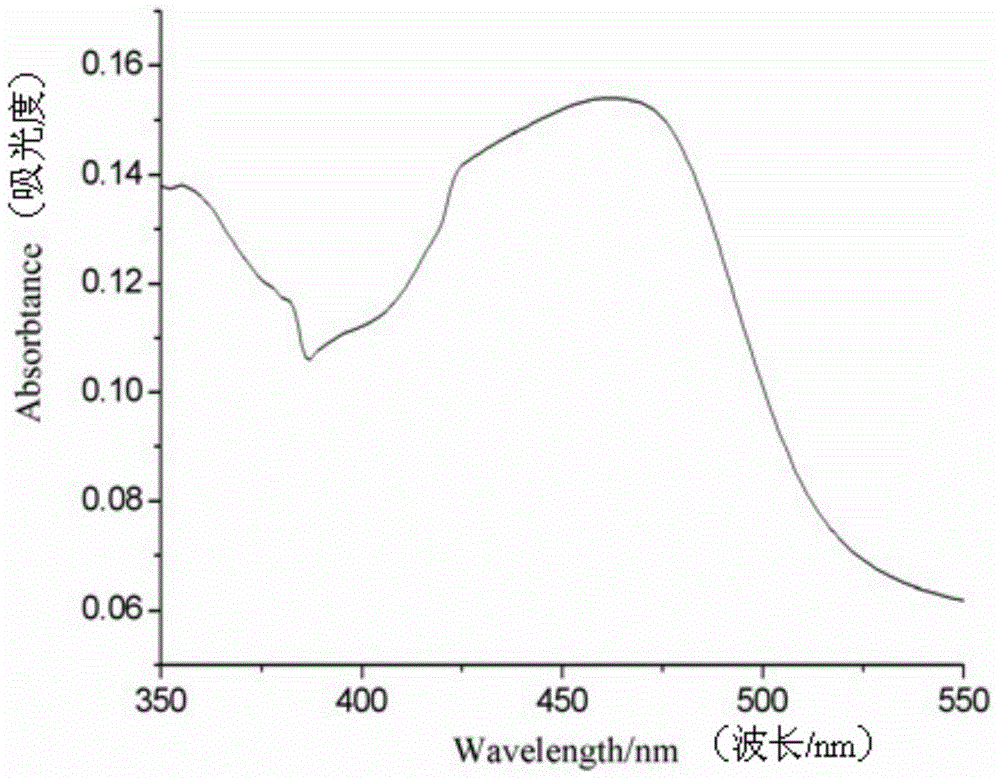
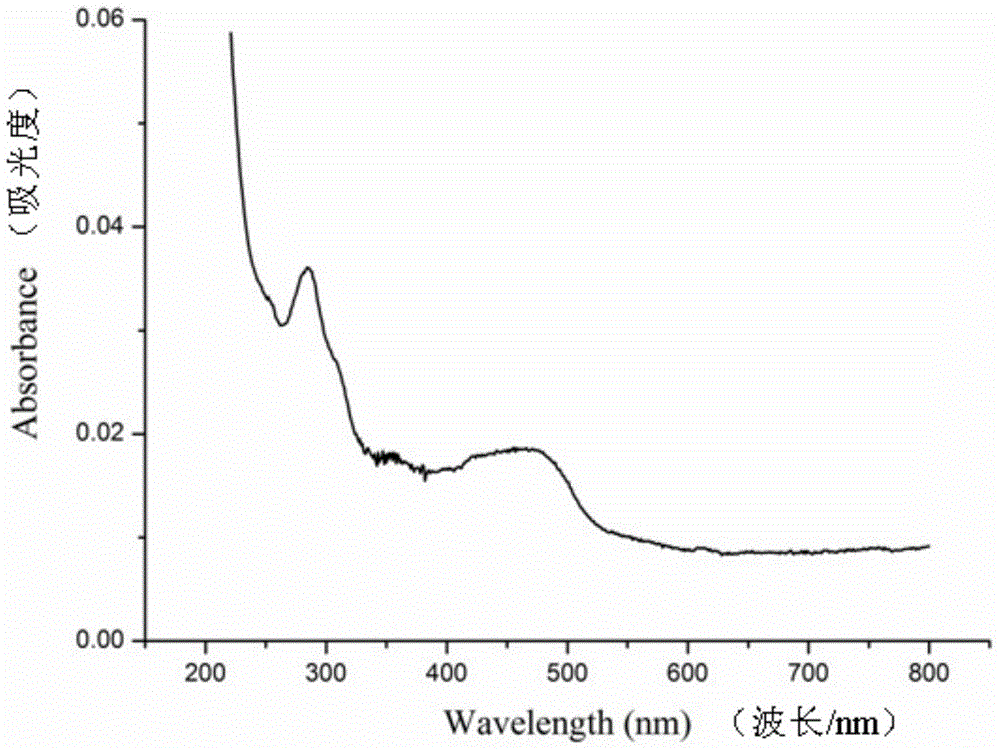
[0099] (Ⅲ) Signal detection: put the product of step (Ⅱ) into a Roche Elecsys 2010 electrochemiluminescence automatic immunoassay analyzer to detect the electrochemiluminescence signal, and record the data. Experimental results such as Figure 4 shown. The results showed that the electrochemiluminescence signal was significantly enhanced by using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com