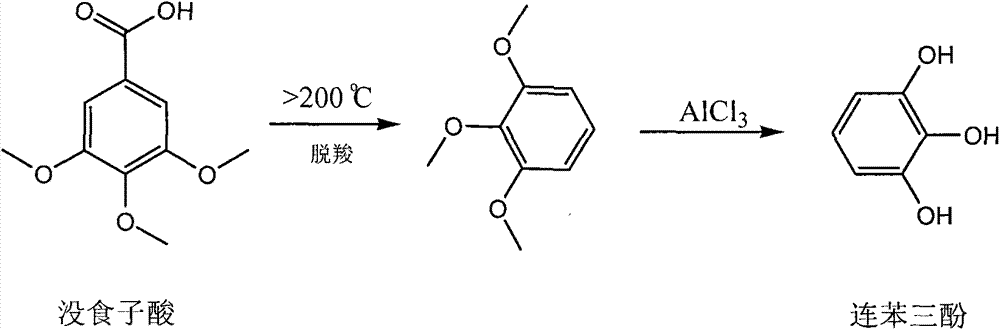
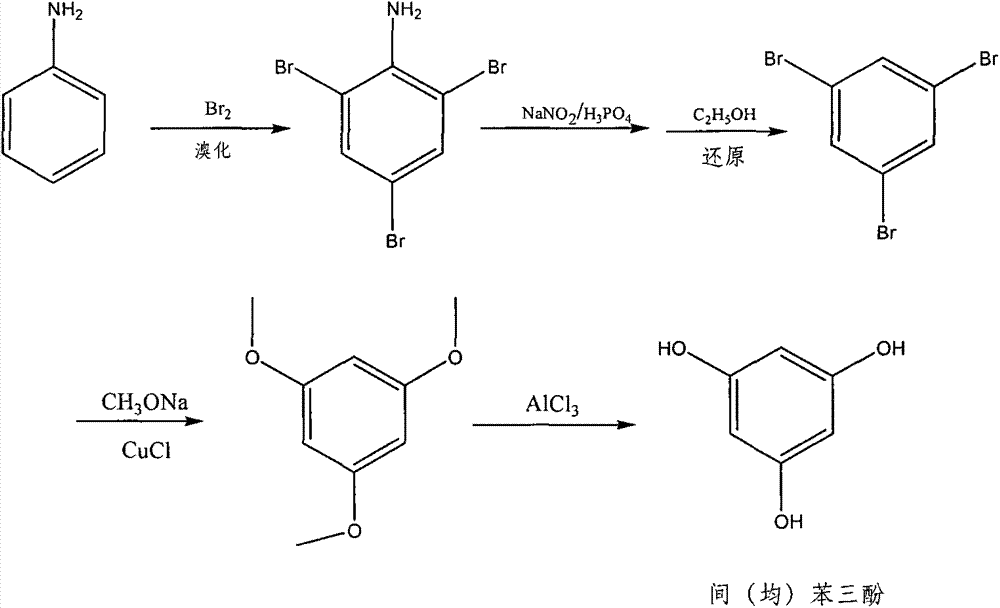
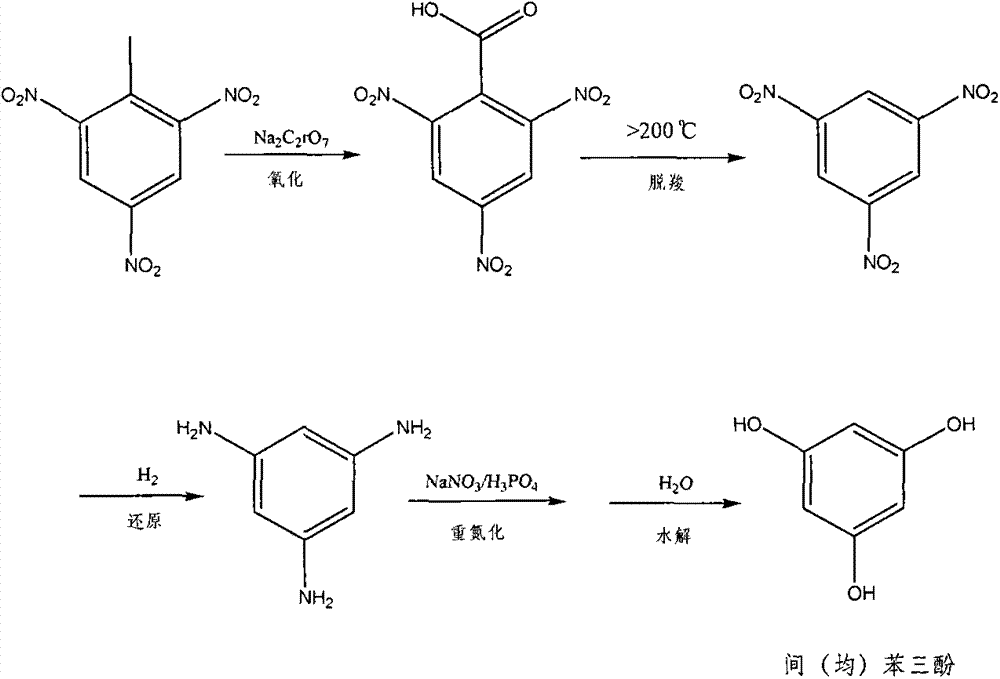
Method for preparing pyrogallol and phloroglucinol
A technology of pyrogallol and pyrogallol, applied in the field of marine chemical and pharmaceutical intermediates, can solve the problems of high price, large pollution and many influencing factors, achieve good activity and stability, reduce production costs, and facilitate industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of pyrogallol
[0032] Add 200g of 3,4,5-trimethoxybenzaldehyde, 300mL of tetrahydrofuran, and 6g of modified framework nickel into a 1L autoclave. After airtight, replace with nitrogen and hydrogen for 3 times respectively, fill with nitrogen to 2MPa, and then raise the temperature to 165 °C, adjust the pressure inside the kettle to 3.5 MPa, maintain this condition for 1.5 hours, and analyze by gas chromatography, the conversion rate of raw materials is 100%, and the selectivity of 1,2,3-trimethoxybenzene is 93%. The solution is extracted from the kettle, the catalyst is left in the kettle and washed with tetrahydrofuran for 2-3 times, the washed tetrahydrofuran is combined with the extracted solution, and the combined solution is rectified to collect 1,2,3-trimethoxybenzene fraction to obtain 155g, Yield 90.6%.
[0033] Add 50 g of 1,2,3-trimethoxybenzene, 220 g of aluminum trichloride, and 200 mL of chloroform into a 1000 mL three-neck fl...
Embodiment 2
[0035] The synthesis of embodiment 2 pyrogallol
[0036] Add 200g of 3,4,5-trimethoxybenzaldehyde, 300mL of cyclohexane, and 4g of modified framework nickel into a 1L autoclave, seal it and replace it with nitrogen and hydrogen for 3 times, fill it with nitrogen to 2MPa, and then raise the temperature To 165°C, adjust the pressure inside the kettle to 3.5 MPa, maintain this condition for 3 hours, sample gas chromatography analysis, the conversion rate of raw materials is 100%, and the selectivity of 1,2,3-trimethoxybenzene is 92%. Extract the solution from the kettle, leave the catalyst in the kettle and wash it with cyclohexane for 2-3 times, combine the washed cyclohexane with the extracted solution, and rectify the combined solution to collect 1,2,3-trimethoxybenzene The fraction was 152g, and the yield was 88.7%.
[0037] Add 50g of 1,2,3-trimethoxybenzene, 250g of aluminum trichloride, and 250mL of dichloromethane into a 1000mL three-neck flask with reflux condenser, dro...
Embodiment 3
[0038] Embodiment 3: the synthesis of pyroglucinol
[0039]Add 200g of 2,4,6-trimethoxybenzaldehyde, 300mL of cyclohexane, and 3g of modified framework nickel into a 1L autoclave, seal it and replace it with nitrogen and hydrogen for 3 times respectively, fill it with nitrogen to 2MPa, and then raise the temperature To 165°C, adjust the pressure inside the kettle to 3.5 MPa, maintain this condition for 4 hours, sample gas chromatography analysis, the conversion rate of raw materials is 100%, and the selectivity of 1,3,5-trimethoxybenzene is 95%. Extract the solution from the kettle, leave the catalyst in the kettle and wash it with cyclohexane for 2-3 times, combine the cyclohexane washing solution with the extracted solution, and rectify the combined solution to collect 1,3,5-trimethoxybenzene The fraction was 156g, and the yield was 90.9%.
[0040] Add 50g of 1,3,5-trimethoxybenzene, 240g of aluminum trichloride, and 300mL of chlorobenzene into a 1000mL three-neck flask equ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com