Method for preparing aromatic aldehyde by catalytic oxidation of toluene compound
A technology for catalytic oxidation and compounds, which is applied in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc. It can solve the problems of difficult to control oxidation depth, pollute the environment, equipment corrosion, etc., and achieve reaction selectivity and yield. The effect of high reaction selectivity and yield, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
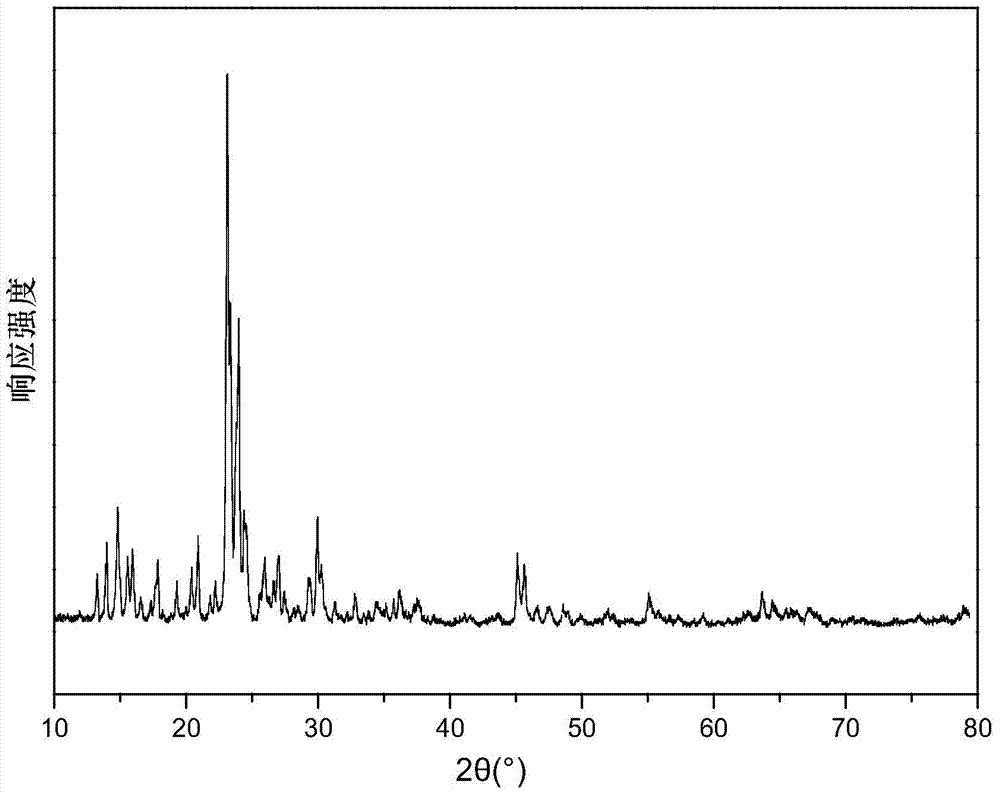
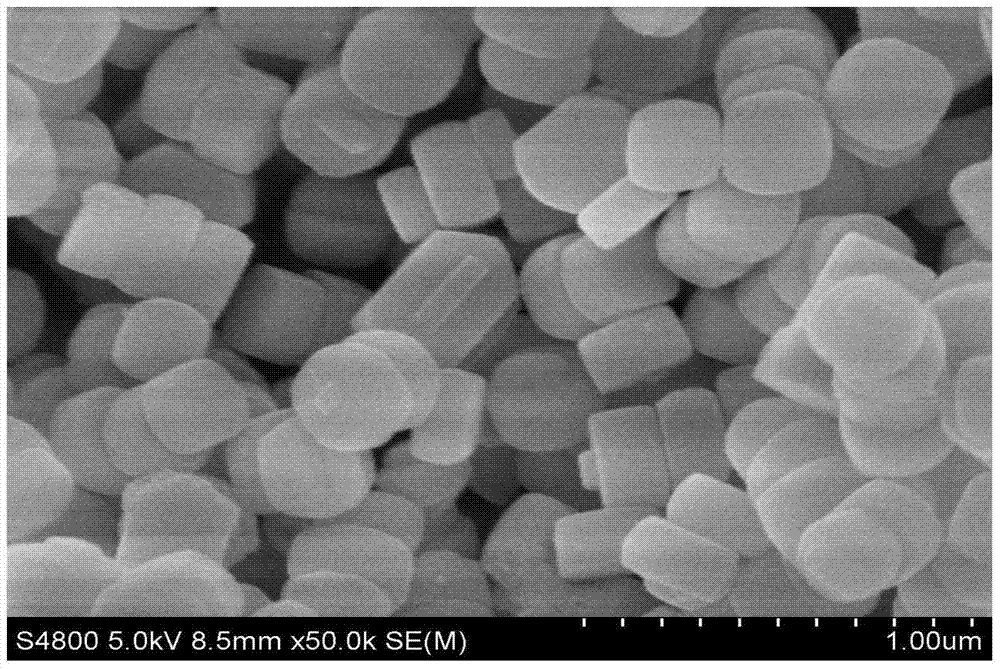
Image
Examples
Embodiment 1
[0025] Add 0.2g of cobalt isomorphously substituted ZSM-5 molecular sieve, 0.5g of potassium bromide, 1mL of toluene, and 10mL of glacial acetic acid into the three-necked flask in sequence, feed oxygen at a flow rate of 100mL / min, and react under stirring at 100°C After 12 hours, the gas chromatography-mass spectrometry analysis results showed that the product was the target product benzaldehyde, and the gas chromatography analysis results showed that the conversion rate of toluene was 90.2%, and the selectivity and yield of benzaldehyde were 78.4% and 70.7%, respectively.
Embodiment 2
[0027] Add 0.2 g of zirconium isomorphic substituted ZSM-5 molecular sieves, 2.0 g of hydrogen bromide with a mass fraction of 30.0%, 1 mL of o-chlorotoluene, and 10 mL of glacial acetic acid into the three-necked flask, and feed oxygen at a flow rate of 100 mL / min. After reacting at 80°C for 14h under stirring, the gas chromatography-mass spectrometry analysis results showed that the product was the target product o-chlorobenzaldehyde, and the gas chromatography analysis results showed that the conversion rate of o-chlorotoluene was 91.2%, and the selection of o-chlorobenzaldehyde The yield and yield were 68.6% and 62.6%, respectively.
Embodiment 3
[0029] Add 0.3 g of ZSM-5 molecular sieves substituted by manganese isomorphs, 2.0 g of hydrogen bromide with a mass fraction of 40%, 1 mL of p-bromotoluene, and 10 mL of acetonitrile in the three-necked flask, and feed oxygen at an oxygen flow rate of 160 mL / min. Under 80 DEG C, react 8h, gas chromatography-mass spectrometry analysis result shows that product is target product p-bromobenzaldehyde, and gas chromatography analysis result shows that the conversion rate of p-bromobenzaldehyde is 94.4%, the selectivity of p-bromobenzaldehyde and The yields were 71.3% and 67.3%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com