Zwitterion-containing water-soluble cross-linking agent as well as preparation method and application thereof
A solvent and reaction technology, applied in the preparation of peptides, organic compounds, sulfonates, etc., can solve the problems of limiting the application of zwitterionic monomers, and achieve excellent anti-biological pollution characteristics, high purity, and side effects little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, compound shown in synthetic formula IV
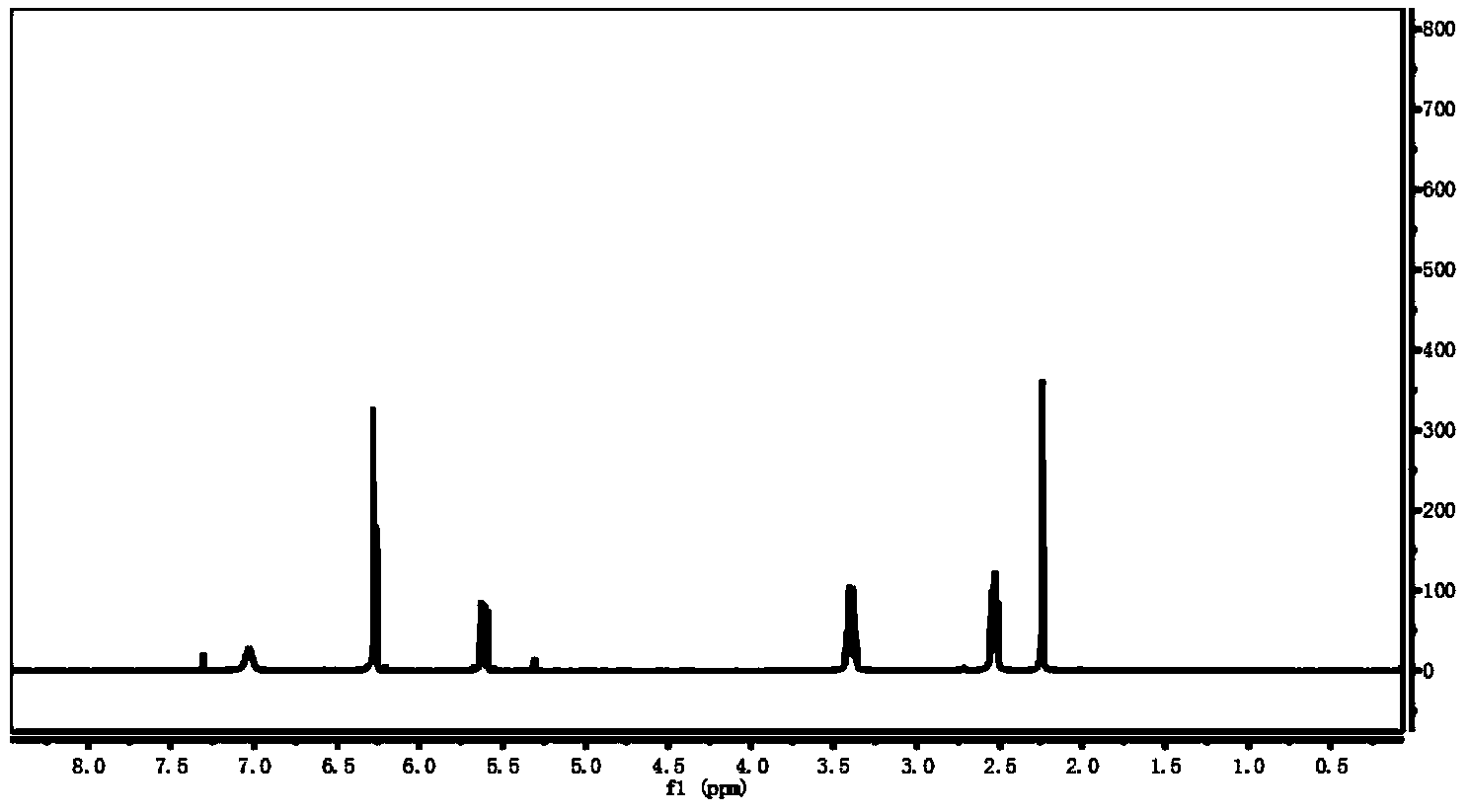
[0063] 1.17g (0.01mol) was attributed to the N-methyl-2,2'-diaminodiethylamine of the compound shown in formula II (at this time R 1 is methyl, R 2 is ethyl, R 3 is ethyl) in a 100mL single-necked flask, add 30mL of solvent dichloromethane, and add 5mL of 6mol / L sodium hydroxide aqueous solution into the reaction flask (sodium hydroxide is used here as an acid-binding agent to remove the hydrogen chloride generated during the reaction to ensure the reaction The smooth progress), magnetic stirring, 2.7g (0.03mol) acryloyl chloride belonging to the compound shown in formula III (at this time R 4 (for hydrogen) was slowly added to the above solution, and the amidation reaction was continued at 0°C for 5h. After the reaction, the solution is placed in a separatory funnel, and the lower layer solution is rotary evaporated to obtain the compound shown in the formula IV. The structure is as follows, and its hydrogen nuc...
Embodiment 2
[0066] Embodiment 2, compound shown in synthetic formula I
[0067] 2.25 g (0.01 mol) of the compound shown in Formula IV obtained in Example 1 was dissolved in 20 mL of solvent acetonitrile, and 1.83 g (0.015 mol) of 1-3' propane sultone belonging to the compound shown in Formula V was added (at this time R 5 methylene) and 4 mg of hydroquinone (inhibitor), reacted at 30°C for 8 hours to obtain a viscous solid precipitate, then repeatedly cleaned the solid precipitate with acetonitrile, and removed excess acetonitrile by rotary evaporation to obtain a white Powder precipitation.
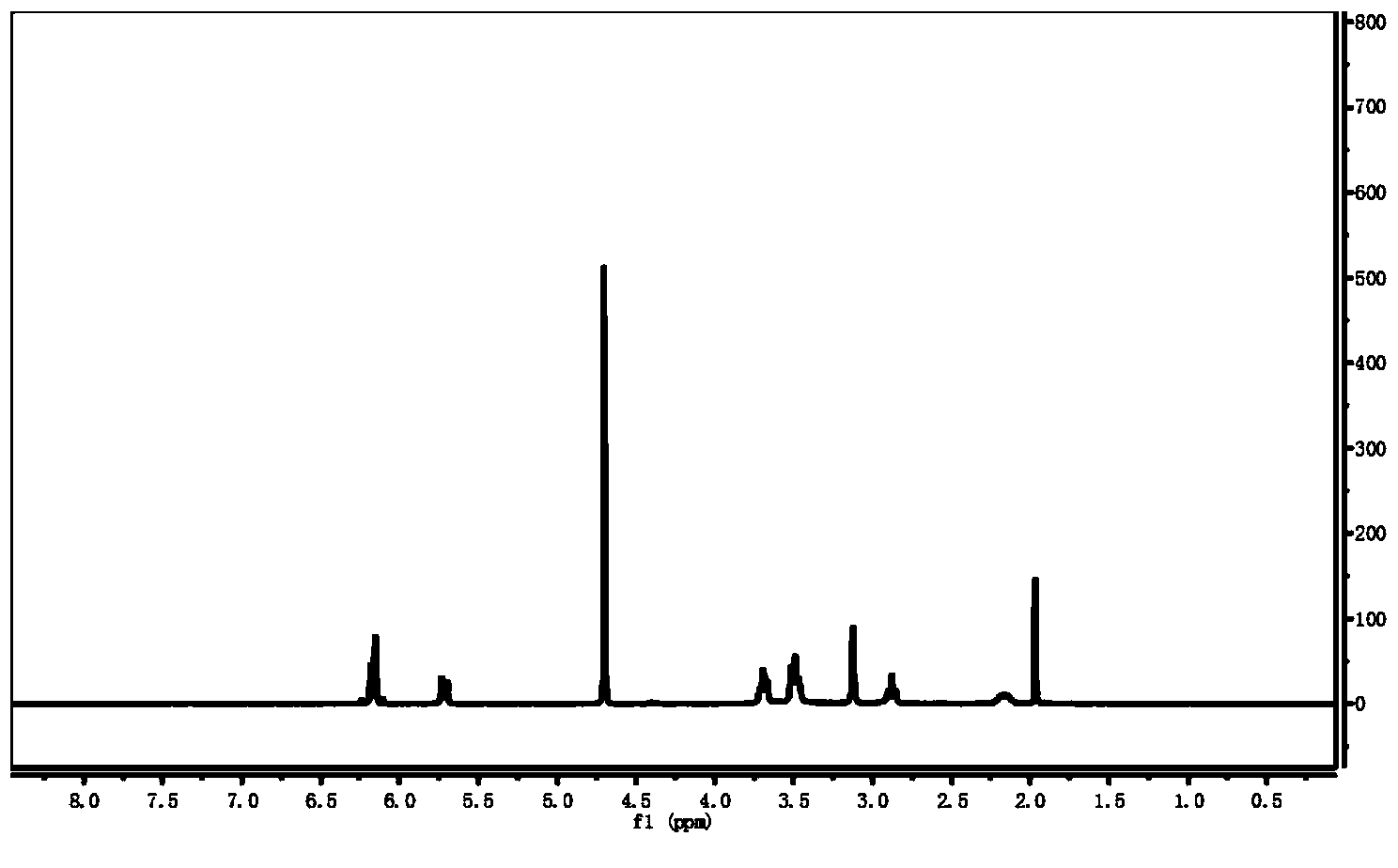
[0068] Proton NMR spectrum analysis, the structure of the product is shown below, and its proton nuclear magnetic resonance spectrum is as attached figure 2 shown.
[0069]
[0070] Among them, attached figure 2 1 In the H NMR spectrum, the characteristic peaks with a chemical shift of 5.69-6.18ppm are the proton peaks at a and b; the characteristic peaks with a chemical shift of 3.47-3.51p...
Embodiment 3
[0074] Embodiment 3, compound shown in synthetic formula IV
[0075] According to the steps of Example 1, only 30mL of dichloromethane was replaced by 40mL of dichloromethane, and 2.7g (0.03mol) of acryloyl chloride was replaced by 0.9g (0.01mol) of acryloyl chloride belonging to the compound represented by formula III, and amidation reaction The temperature was replaced by 0°C to 25°C, and the time was replaced by 5 hours to 1 hour to obtain the compound shown in formula IV;
[0076] The structural verification data of this product is not substantially different from that of Example 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com