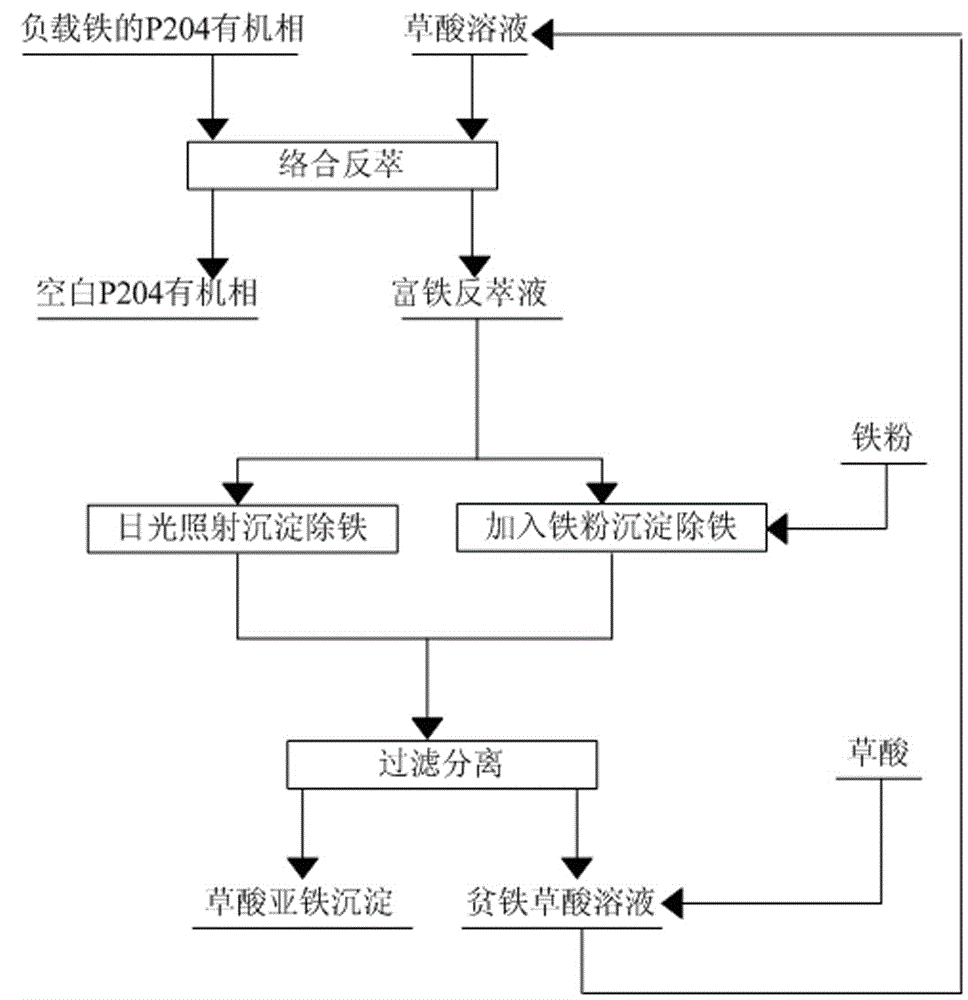
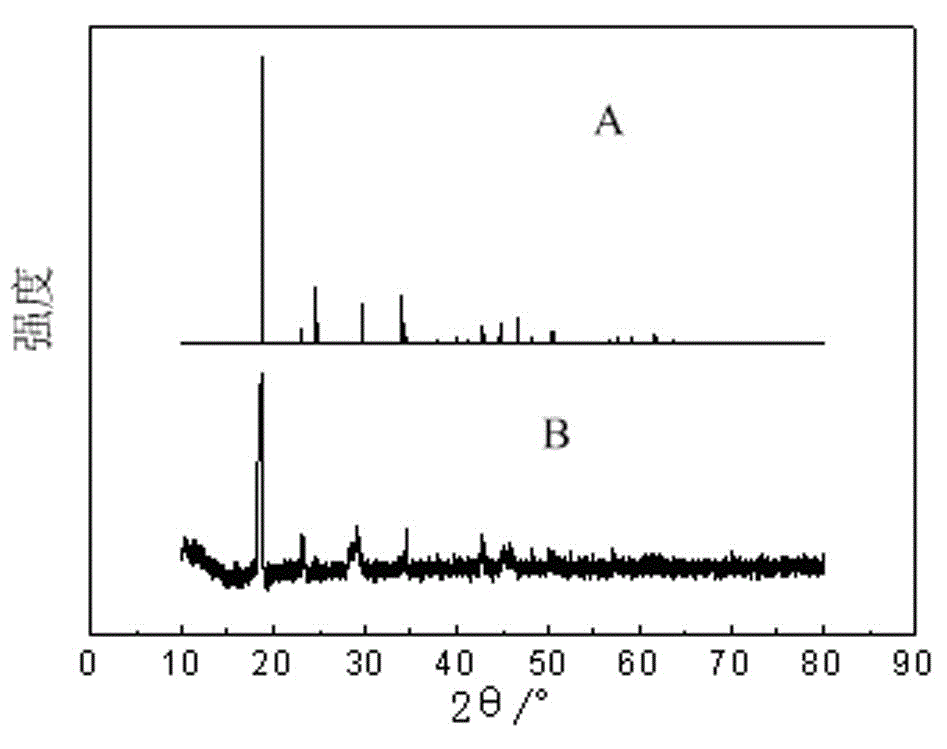
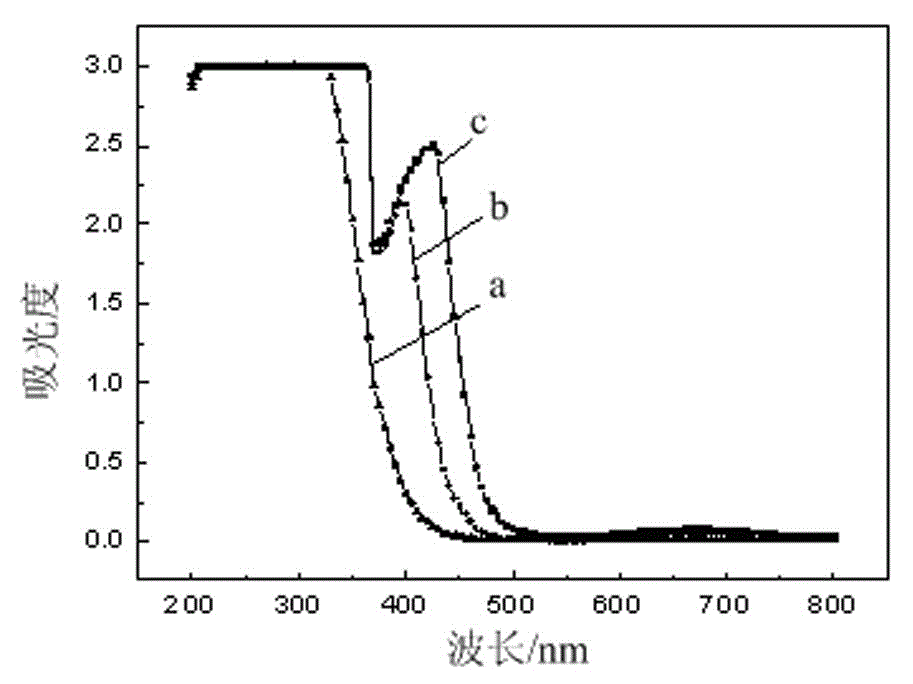
A method for back-extracting iron-loaded p204 organic phase and back-extraction solution for removing iron
A stripping liquid and organic phase technology, applied in the field of hydrometallurgy, can solve the problems of being unable to get rid of the use, high acid and alkali consumption, and reduced effect, and achieve the effect of solving the dependence on natural weather conditions, low cost, and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The volume of extractant P204 accounts for 10% in the P204 organic phase of loaded iron, and the content of Fe(III) is at 0.51g / L;
[0032] with H 2 C 2 o 4 The oxalic acid solution with a mass concentration of 4% was used as the stripping agent, and the iron-loaded P204 organic phase was mixed with the stripping agent at a volume ratio of 1:1 to perform a first-stage stripping. The stripping temperature was 20°C. After the stripping was completed, Obtain iron-rich stripping solution and blank P204 organic phase; the stripping rate of iron in the iron-loaded P204 organic phase is 99.5%;
[0033] The iron-rich back-extraction solution is subjected to photodecomposition and precipitation to remove iron by sunlight irradiation, and the sunlight irradiation time is 4 hours;
[0034] Filter the material after precipitation and iron removal to separate the solid phase and liquid phase;
[0035] The separated liquid phase is an iron-poor oxalic acid solution, which is recycl...
Embodiment 2
[0039] The volume of extractant P204 accounts for 40% in the P204 organic phase of loaded iron, and the content of Fe(III) is at 4.89g / L;
[0040] with H 2 C 2 o 4 The oxalic acid solution with a mass concentration of 6% is used as the stripping agent, and the iron-loaded P204 organic phase is mixed with the stripping agent at a volume ratio of 0.5:1 to perform a first-stage stripping. The stripping temperature is 30 ° C. After the stripping is completed Obtain iron-rich stripping solution and blank P204 organic phase; the stripping rate of iron in the iron-loaded P204 organic phase is 99.8%;
[0041] The iron-rich back-extraction solution is subjected to photodecomposition and precipitation to remove iron by sunlight irradiation, and the sunlight irradiation time is 6 hours;
[0042] Filter the material after precipitation and iron removal to separate the solid phase and liquid phase;
[0043] The separated liquid phase is an iron-poor oxalic acid solution, which is recyc...
Embodiment 3
[0047] The volume of extractant P204 in the P204 organic phase of loaded iron accounts for 30%, and the content of Fe(III) is at 2.61g / L;
[0048] with H 2 C 2 o 4 The oxalic acid solution with a mass concentration of 6% is used as the stripping agent, and the P204 organic phase loaded with iron is mixed with the stripping agent at a volume ratio of 3:1 to carry out 3-stage stripping, and the 3-stage stripping is countercurrent stripping, stripping The temperature is 40°C. After the stripping is completed, an iron-rich stripping solution and a blank P204 organic phase are obtained; the stripping rate of iron in the iron-loaded P204 organic phase is 99.6%;
[0049] The iron-rich back-extraction solution is subjected to photodecomposition and precipitation to remove iron by sunlight irradiation, and the sunlight irradiation time is 12 hours;
[0050] Filter the material after precipitation and iron removal to separate the solid phase and liquid phase;
[0051] The separated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com