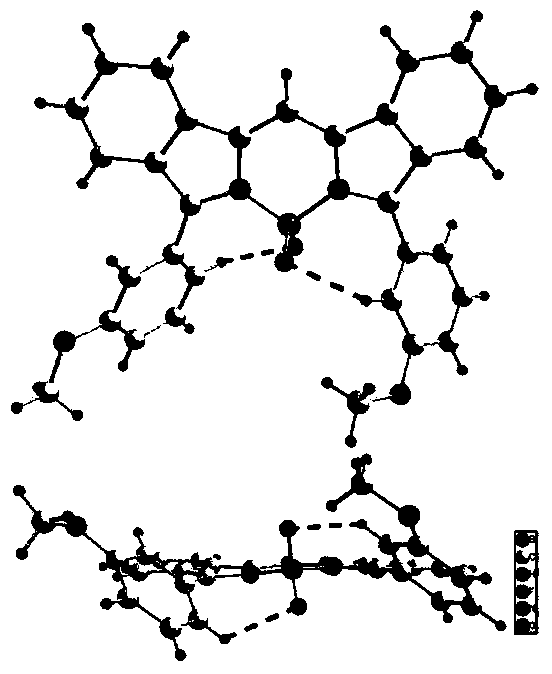
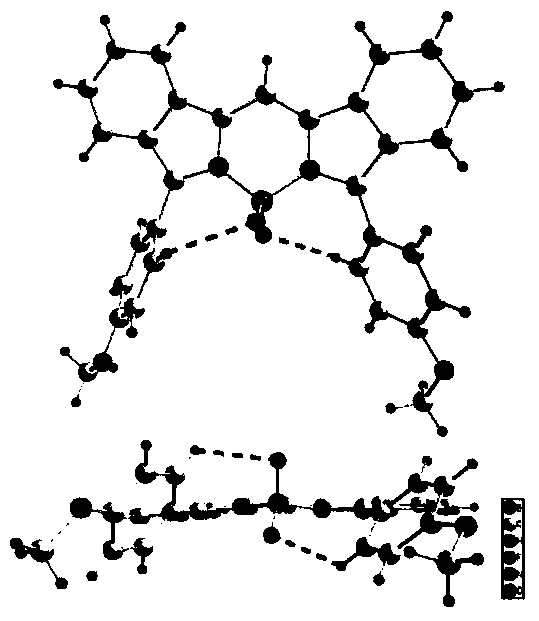
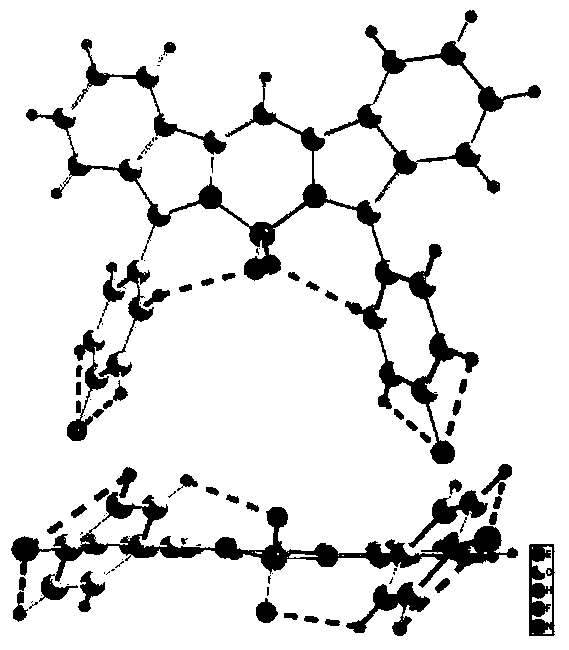
Near-infrared fluorine-boron dipyrrole fluorochrome and preparation method thereof
A technology of fluoroboridipyrrole and fluorescent dyes, which is applied in the field of near-infrared fluoroboridipyrrole fluorescent dyes and its preparation, can solve the problems of high activity and instability at the alfa position, complex synthesis methods, and low yields, and achieve excellent photophysical The effects of chemical properties, excellent product spectral properties, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of Dye 2-2a:
[0050]
[0051] Weigh 400 mg of imine, 532 mg of 3-methoxyphenylboronic acid (2.2e.q.) into a 100 mL Slack reactor, add 15 mL of toluene, 10 mL of 1M Na 2 CO 3 Solution, 2mL ethanol, after solidification in liquid nitrogen environment, vacuumize, argon flow, repeat three times, add catalyst Pd(PhP 3 ) 4 60 mg, vacuum again, argon, repeat three times. After the reactor returned to room temperature, it was placed in an 80°C oil bath and heated for 12h. Point the plate, the imine reaction is complete, and the reaction is over. It was extracted with ethyl acetate, concentrated, and passed through the column to obtain 335 mg of 1-1 yellow powder with a yield of 83.8%. 1 H NMR (300MHz, CDCl 3 )δ9.91(s, 1H), 8.02(t, J=4.8Hz, 2H), 7.50-7.37(m, 4H), 7.26(t, J=7.5Hz, 1H), 7.02(d, J=7.2 Hz,1H),3.92(s,3H); 13 CNMR (75MHz, CDCl 3 )δ173.9,161.0,130.5,127.5,124.4,124.0,122.1,122.0,121.9,120.0,117.6,115.2,115.1,112.9,112.8,55.5.HRMS(ESI)calcd.for C ...
Embodiment 2
[0054]
[0055] Weigh 400 mg of imine, 532 mg of 4-methoxyphenylboronic acid (2.2e.q.), and repeat the above operation to obtain 350 mg of 2-1b yellow powder with a yield of 87.5%. 1 H NMR (300MHz, CDCl3 )δ9.87(s,1H),7.98(d,J=6.9Hz,2H),7.77(d,J=8.1Hz,2H),7.41(s,1H),7.26(s,1H),7.10- 7.07(m,2H),3.90(s,3H); 13 CNMR (75MHz, CDCl 3 )δ173.0,160.4,135.8,135.7,133.2,133.1,129.5,127.4,123.9,123.5,123.0,122.1,121.8,117.4,114.6,55.4. HRMS(ESI)calcd.for C 16 h 13 NO 2 [M+H] + :252.1019,found252.1019.
[0056] In a 50 mL round bottom flask, add 20 mL of CH under argon 2 Cl 2 , add aldehyde (90 mg, 0.5 mmol), and add POCl which has been dissolved in 1 mL of dichloromethane 3 (0.47ml, 5mmol), the solution immediately changed from light yellow to yellow-green and then to green, and the color gradually deepened as the reaction time increased. After reacting for 4 hours, add 1.0 mL of diisopropylamine at room temperature, stir at room temperature for 10 minutes, add 1.2 mL of boron ...
Embodiment 3
[0058]
[0059] Weigh 400 mg of imine and 624 mg of 4-tert-butylphenylboronic acid (2.2e.q.), repeat the above operation to obtain 348 mg of 3-1 yellow powder with a yield of 79%. 1 H NMR (300MHz, CDCl 3 )δ9.90(s, 1H), 8.01(t, 2H), 7.78(d, J=8.1Hz, 2H), 7.57(d, J=8.1Hz, 2H), 7.41(s, 1H), 7.24( s,1H),1.39(s,9H); 13 C NMR (75MHz, CDCl 3 )δ173.1,152.5,134.3,132.4,127.7,127.4,127.3,126.4,123.9,123.7,122.1,121.8,117.5,34.9,31.2.HRMS(ESI)calcd.for C 19 h 19 NO[M+H] + :278.1539,found278.1539.
[0060] In a 50 ml round bottom flask, add 20 ml of CH under argon 2 Cl 2 , add aldehyde (90 mg, 0.5 mmol), and add POCl which has been dissolved in 1 mL of dichloromethane 3 (0.47ml, 5mmol). After reacting for 4 hours, 1.0ml of diisopropylamine was added at room temperature, and after stirring at room temperature for 10 minutes, 1.2mL of boron trifluoride ether was added, and the round bottom flask was sealed. After stirring at room temperature for 2 h, extraction, drying, concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com