New xanthine oxidase inhibitor compound and pharmaceutical composition thereof
A compound and pharmaceutical technology, applied in the field of a new class of xanthine oxidase inhibitor compounds and their pharmaceutical compositions, can solve the problems of high incidence, abnormal liver function and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
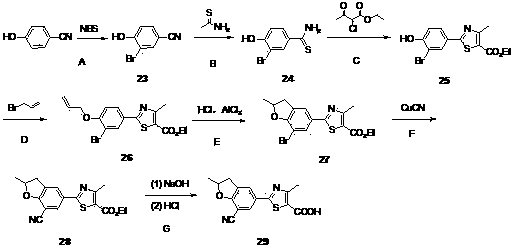
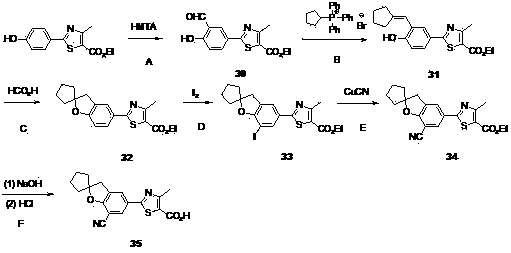
Embodiment 1
[0089] 2-(7-cyano-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-4-methyl-thiazole-5-carboxylic acid ( 8 )Synthesis
[0090]
[0091] Step A: Thioacetamide (17.0 g, 226.7 mmol) and p-hydroxybenzonitrile (18.0 g, 151.1 mmol) were added to concentrated hydrochloric acid (470 mL), and the resulting mixture was stirred at 50 °C for 3 hours, and TLC analysis indicated a reaction Finish. The reaction solution was cooled to 0°C, filtered, the filter cake was washed with a small amount of water, and dried in vacuum to obtain a yellow powder p-hydroxythiobenzamide ( 1 ) (26.0g). The product was directly used in the next reaction without purification. MS (EI, m / z): 154.1 [M+H] + .
[0092] Step B: Compound 1 (25.0 g, 163.2 mmol) and ethyl 2-chloroacetoacetate (26.5 g, 161.0 mmol) were added to absolute ethanol (75 mL), and the resulting mixture was stirred under reflux for 3 hours, and TLC analysis indicated that the reaction was complete. The reaction solution was cooled to 0°C, a...
Embodiment 2
[0104] 2-(7-iodo-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-4-methyl-thiazole-5-carboxylic acid ( 9 )Synthesis
[0105]
[0106] compound 6 According to the example 1 The method in experimental step H is hydrolyzed and acidified to give 2-(7-iodo-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-4-methyl-thiazole- 5-Formic acid ( 9 ).
[0107] 1 H NMR (DMSO- d 6 , 400MHz) δ 8.07 (d, J = 1.6 Hz, 1H), 7.78 (d, J = 1.6 Hz, 1H), 3.21 (s, 2H), 2.64 (s, 3H), 1.47 (s, 6H). MS (EI, m / z): 413.9 [M-H] - .
Embodiment 3
[0109] 2-(7-cyano-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-4-trifluoromethyl-thiazole-5-carboxylic acid ( 19 )Synthesis
[0110]
[0111] Step A: Dissolve methylparaben (2.0 g, 13.1 mmol) in DMF (14 mL), then add anhydrous potassium carbonate (2.18 g, 15.8 mmol), potassium iodide (0.2 g, 1.20 mmol) and 3 - Chloro-2-methyl-1-propene (1.78 g, 19.7 mmol), and the resulting mixture was stirred at 80°C overnight. Cool to room temperature, add water (60 mL), extract with ethyl acetate (30 mL×3), wash the combined organic phase with water (30 mL), and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by silica gel column (200~300 mesh silica gel, ethyl acetate:petroleum ether=1:10 elution) to obtain 4-(2-methyl-allyloxy)-benzoic acid methyl ester( 10 ) (2.7 g). The yield was 99.5%.
[0112] Step B: Compound 10 (2.7 g, 13.1 mmol) was dissolved in NMP (18 mL), the reaction solution was stirred overnight at 200 °...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com