Heterozygous antibacterial and antiviral polypeptide as well as preparation method and application thereof
An antiviral and hybrid technology, applied in the fields of genetic engineering and biopharmaceuticals, can solve the problems of severe hemolysis, achieve the effects of reducing toxicity and hemolysis, antiviral biological characteristics, and resistance to drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
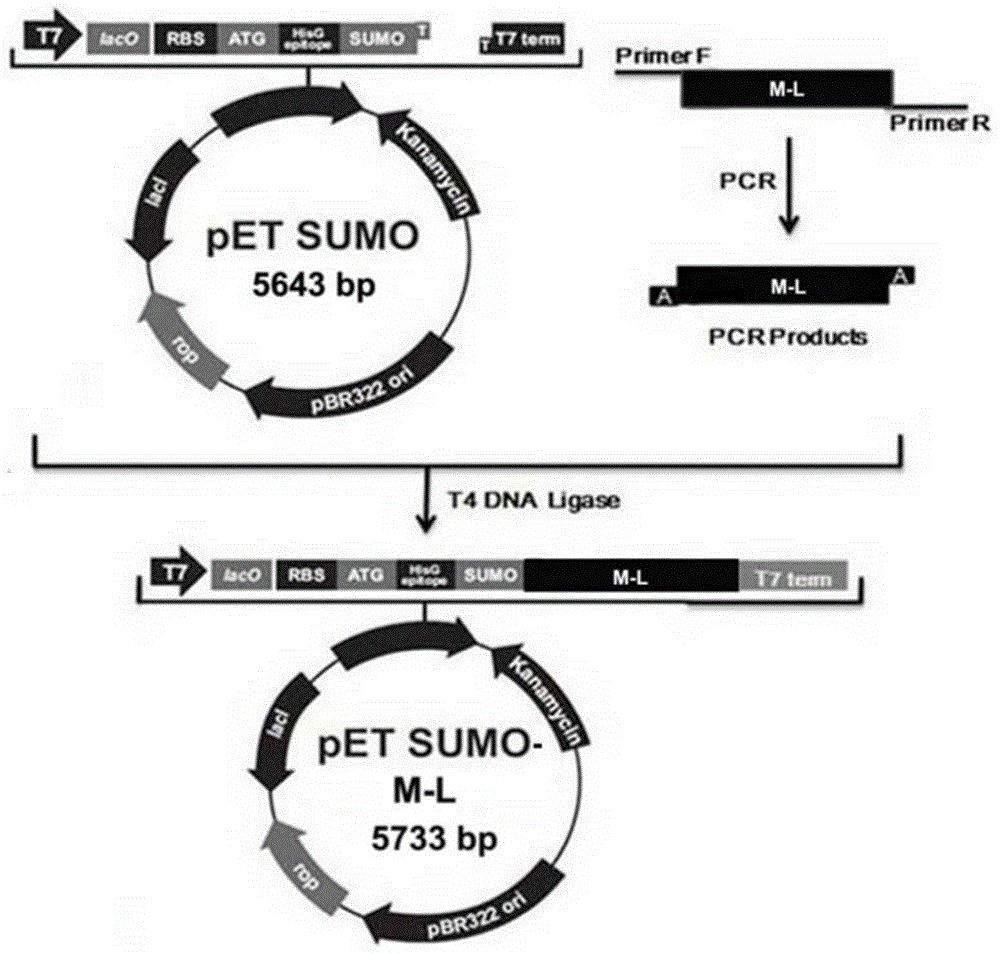
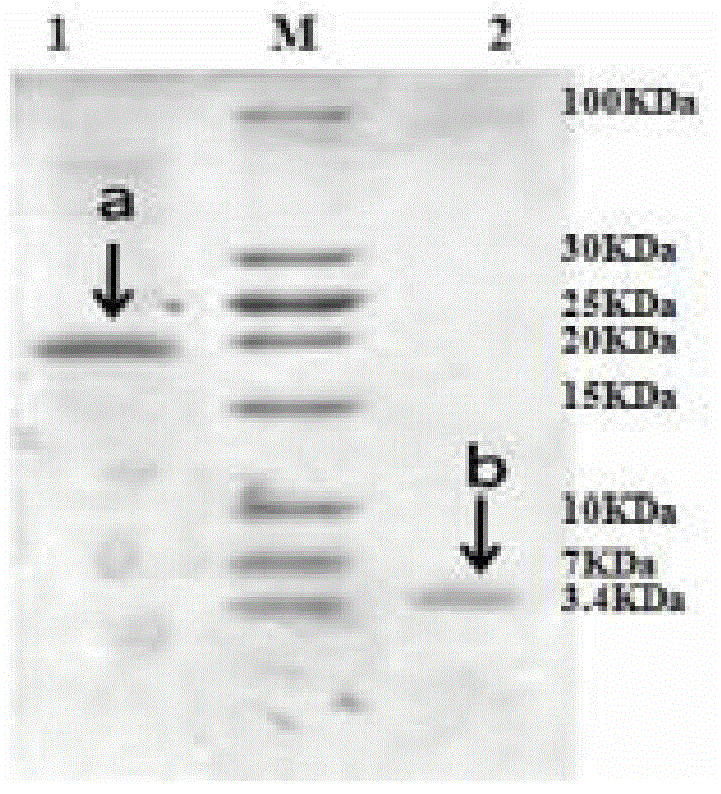
[0037] Example 1 Preparation of hybrid antibacterial and antiviral polypeptide M-L
[0038] Escherichia coli Mach1 TM -T1 R , E. coli BL21 and expression vector pETSUMO (Cat. No.: inv-k300-01) were purchased from Invitrogen.
[0039] Escherichia coli Mach1 TM -T1 R A single colony of BL21 and BL21 was inoculated in 3-5ml LB (1% tryptone, 0.5% yeast powder, 1% sodium chloride) liquid medium, cultured with shaking at 37°C for about 12 hours, according to 1:100-1:50 Proportionally inoculate in 100ml LB liquid medium, culture with shaking at 37℃ for 2-3 hours to OD 600 = About 0.5. The bacterial suspension was inoculated into 100ml LB liquid medium at a ratio of 1:100-1:50, cultured with shaking at 37°C for 2-3 hours to OD 600 = About 0.5, stop the culture. The culture solution was transferred to a centrifuge tube, placed on ice for 10 minutes, and then centrifuged at 3000g for 10 minutes at 4°C to collect the bacteria. Add pre-cooled 0.05mol / L CaCl to the bacteria 2 The cells were g...
Embodiment 2
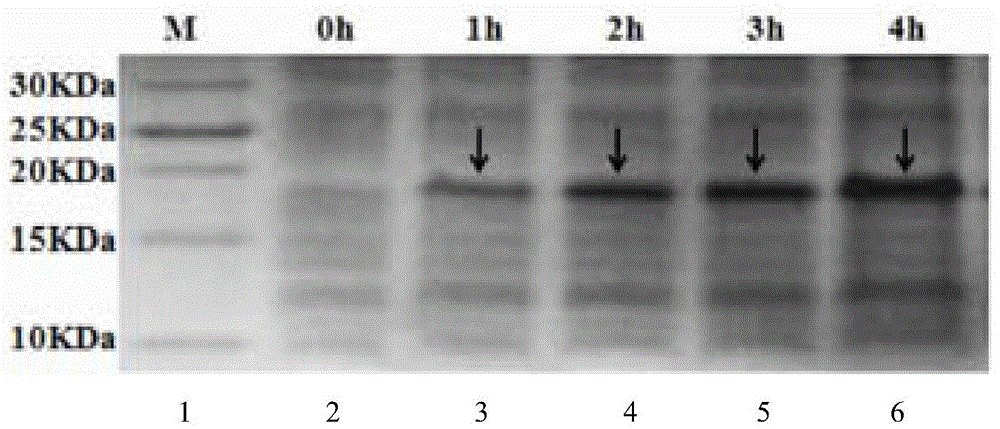
[0048] Example 2 Thermal stability test of polypeptide M-L
[0049] Take 1ml each of the purified recombinant protein and place them in a water bath at 40℃, 60℃, 80℃ and 100℃ for 10, 20 and 30 minutes respectively. Use the untreated recombinant protein as a control and use standard Staphylococcus aureus as an indication Bacteria, the bacteriostatic effect of the samples before and after treatment was measured by the diameter of inhibition zone method. The experiment was repeated 3 times, and the average value was taken as the measurement result. (Table 1)
[0050] Table 1 Diameter of inhibition zone of recombinant target protein M-L on Staphylococcus aureus after heat treatment
[0051]
[0052] Note: The control is the diameter of the inhibition zone of the recombinant target protein without heat treatment.
[0053] The results in Table 1 show that after different heating temperatures and time treatments, the antibacterial effect of the recombinant target protein is different. When...
Embodiment 3
[0054] Example 3 Inhibition of polypeptide M-L on common bacteria
[0055] Put the filter paper (diameter 6mm) sterilized by dry heat (160℃, 2h) into the recombinant target protein solution (32μg / ml) and soak it for 24h. Use a sterile spreader to dip the test bacteria solution evenly. Spread the ground on the surface of the culture medium to make a plate containing bacteria, take out the fully soaked filter paper and stick it on the plate containing bacteria, stick one piece per petri dish, and place it in a constant temperature incubator at 37°C for 24 hours, observe and measure The diameter of the zone of inhibition. (Table 2)
[0056] Table 2 Diameter of inhibition zone of recombinant target protein against common bacteria
[0057] Bacteria name
[0058] Escherichia coli (CICC1.907)
[0059] It can be seen from Table 2 that the antibacterial peptides have different degrees of antibacterial effect on each strain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com