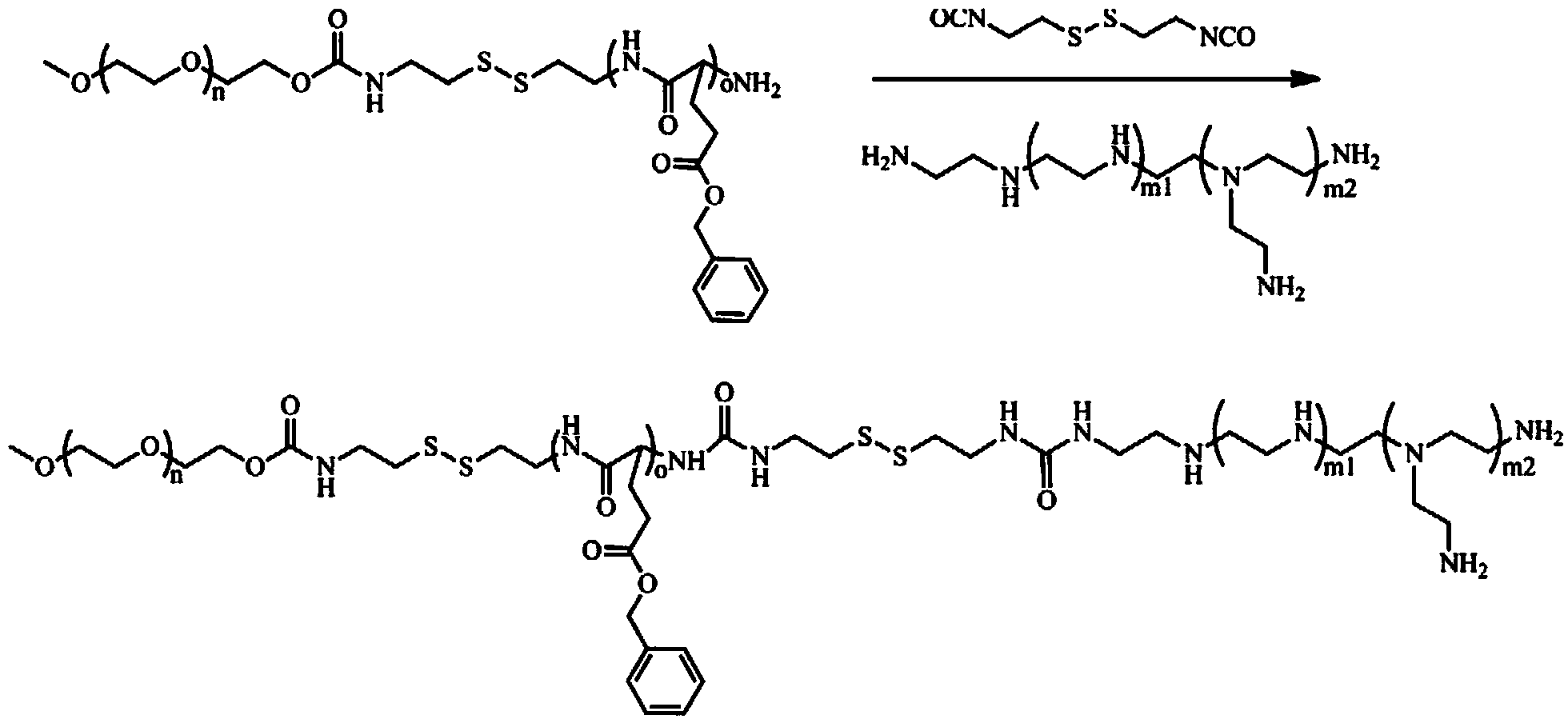
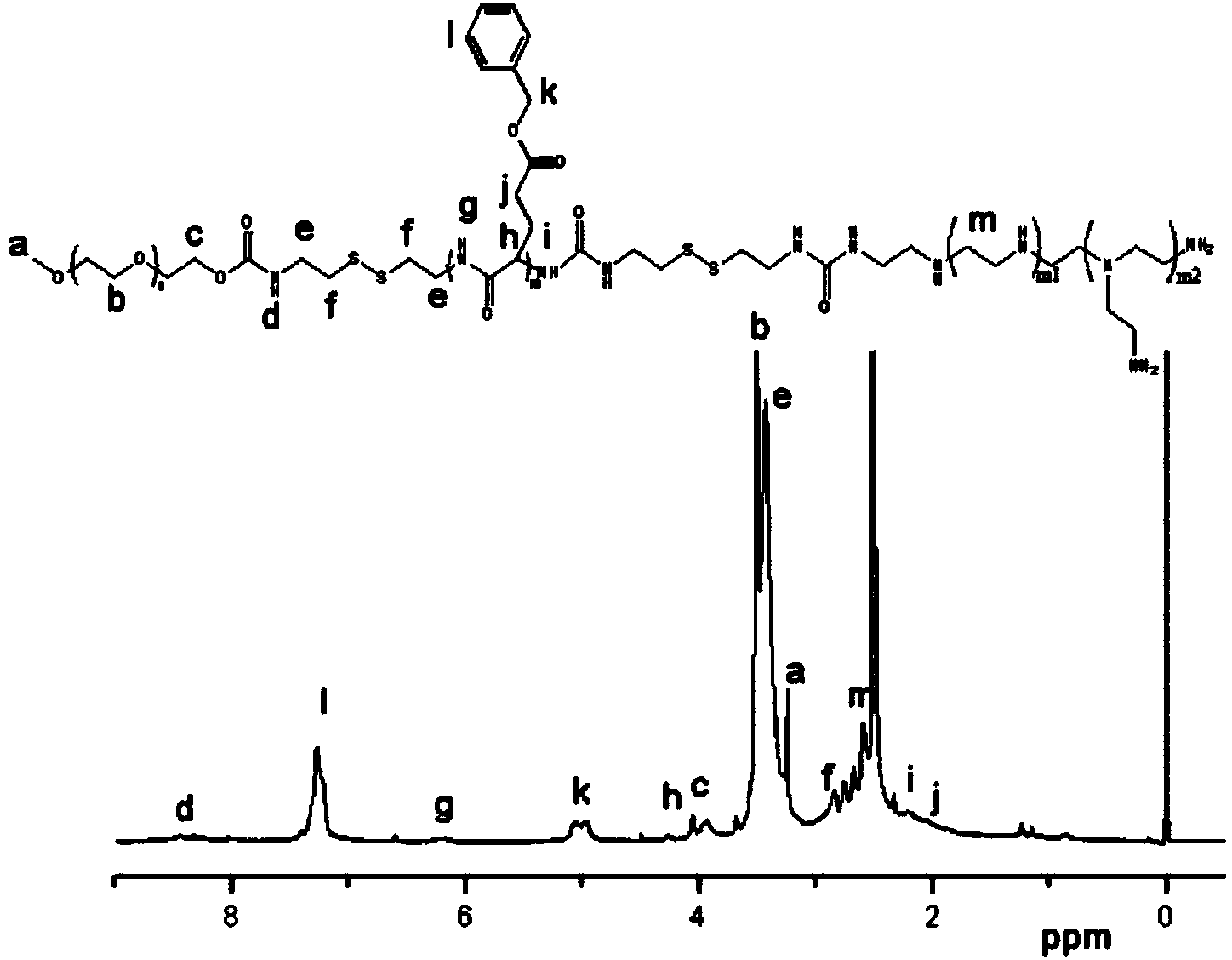
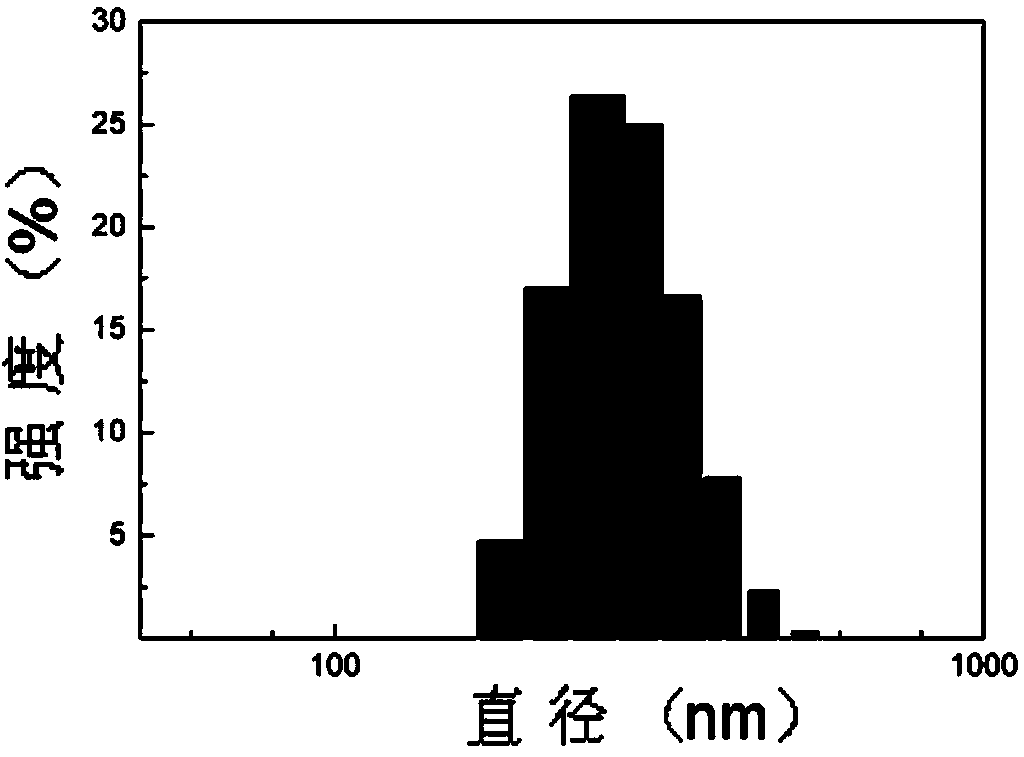
Fully-dissociable type polyethylene glycol-poly(L-glutamate-gamma-benzyl ester)-polyethyleneimine copolymer as well as synthesizing method and application thereof
A polyethyleneimine copolymer and polyethyleneimine technology are applied in the field of completely dissociable polyethylene glycol-polyethyleneimine copolymer and its synthesis, which can solve the problem of repeated medication and inability to combine , patient troubles and other problems, to achieve the effect of increasing the effective concentration of drugs, reducing toxic and side effects, and promoting escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Synthesis of cystaminated methoxypolyethylene glycol (mPEG):
[0047] 1.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 4000Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and store at 85°C under a nitrogen atmosphere. After reacting for 48 hours, the resulting reaction system was precipitated three times in anhydrous n-hexane, and the precipitate was collected and dried in vacuo to obtain mPEG modified with 2,2'-dithiodiethylisocyanate; The molar ratio of polyethylene glycol, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate is 1:0.06:8; dissolve 5g of methoxy-terminated polyethylene glycol per 100ml of anhydrous toluene alcohol;
[0048] 1.2) Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water and react at 60°C for 6 hours. The reaction system is dialyzed in distilled water and freeze-dried to obtain white powder cystaminated endoform Oxypolyethylene glycol; Dissolve 5g of 2,...
Embodiment 2
[0064] 1) Synthesis of cystaminated methoxypolyethylene glycol:
[0065] 1.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 1200Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and react at 85°C under a nitrogen atmosphere After 48 hours, the resulting reaction system was precipitated three times in anhydrous n-hexane, and the precipitate was collected and dried in vacuo to obtain 2,2'-dithiodiethylisocyanate-modified mPEG; wherein, the methoxy-terminated poly The molar ratio of ethylene glycol, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate is 1:0.04:5, and 25g of methoxy-terminated polyethylene glycol is dissolved in 100ml of anhydrous toluene ;
[0066] 1.2) Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water and react at 60°C for 6 hours. The reaction system is dialyzed in distilled water and freeze-dried to obtain white powder cystaminated endoform Oxy-polyethylene glycol; wherein,...
Embodiment 3
[0076] 1) Synthesis of cystaminated methoxypolyethylene glycol:
[0077] 1.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 2000Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and react at 85°C under a nitrogen atmosphere After 48 hours, the resulting reaction system was precipitated three times in anhydrous n-hexane, and the precipitate was collected and dried in vacuo to obtain 2,2'-dithiodiethylisocyanate-modified mPEG; wherein, the methoxy-terminated poly The molar ratio of ethylene glycol, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate is 1:0.02:3, and 10g of methoxy-terminated polyethylene glycol is dissolved in 100ml of anhydrous toluene ;
[0078] 1.2) Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water and react at 60°C for 6 hours. The reaction system is dialyzed in distilled water and freeze-dried to obtain white powder cystaminated endoform Oxy-polyethylene glycol; wherein,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com