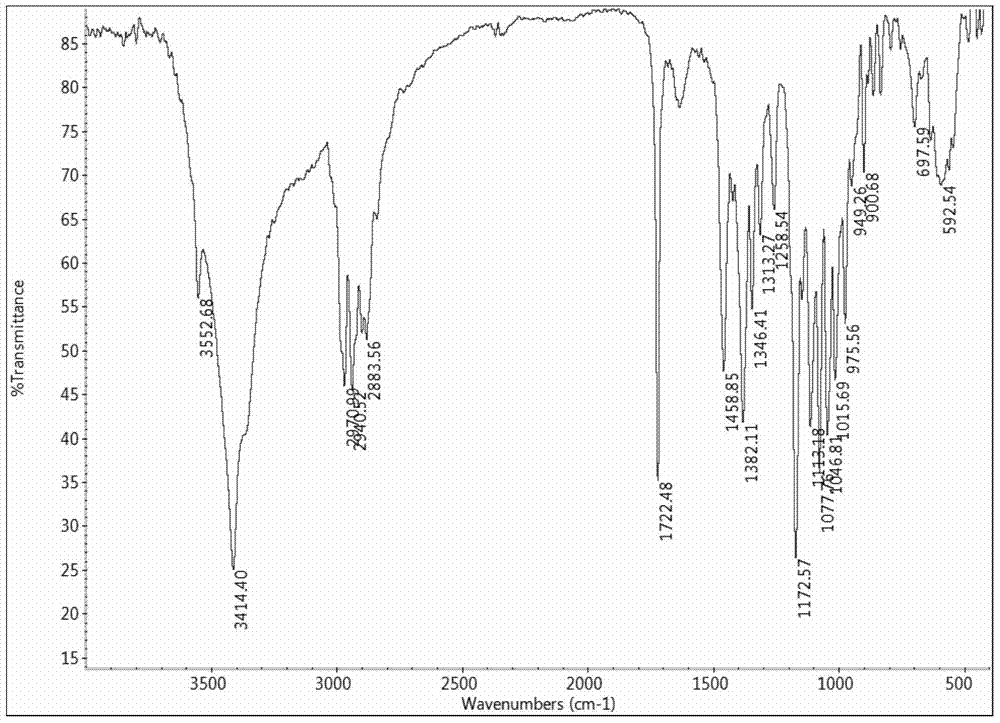
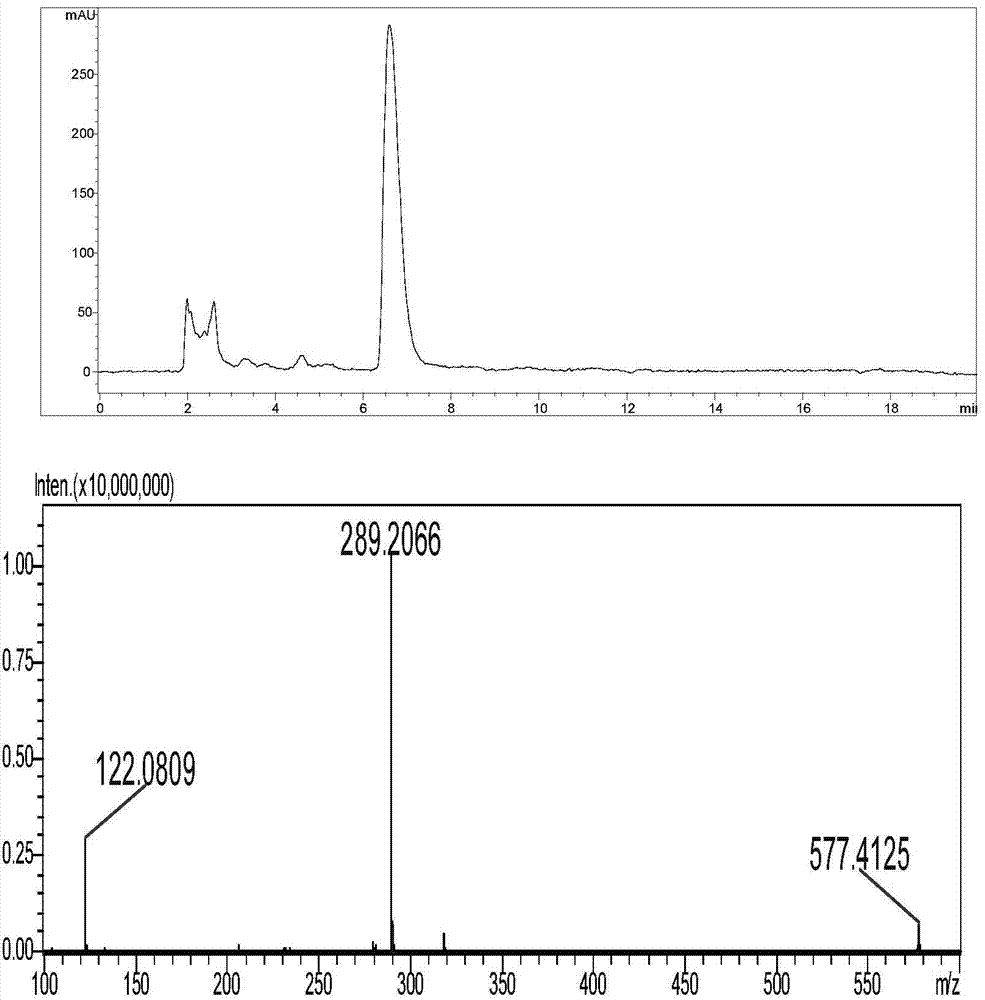
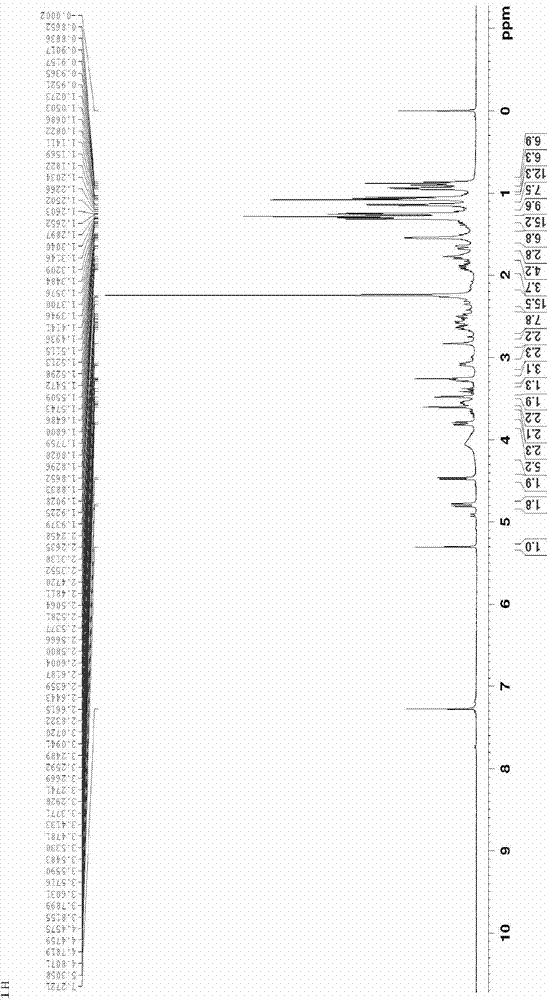
Synthesis method of tulathromycin residue marker
A turamycin and marker technology, which is applied in the field of synthesis of turamycin residue markers, can solve the problems of easy degradation reaction, unstable erythromycin A oxime, and many by-products, etc., and achieves low cost of raw materials. , the effect of easy availability of raw materials and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Dissolve 100 g of erythromycin A(E) oxime in 400 mL of acetone and 400 mL of water, add 50 g of sodium bicarbonate, and under stirring, add 38 g of p-toluenesulfonyl chloride at 0°C (dropped within 10 min), continue to stir for 3 h, and use The course of the reaction was monitored by thin layer chromatography (TLC). After the reaction was completed, the pH of the solution was adjusted to 12 with 20% NaOH, raised to room temperature, and the reaction was stirred for 30 min. At this time, a white solid was formed, suction filtration, and the obtained product was dried in a vacuum drying box at 50 °C for 5 h to obtain red. Mycin A6,9-imine ether 95g, yield>95%, melting point 129~131℃.
Embodiment 2
[0044] Dissolve 19 g of erythromycin A6,9-imine ether in 200 mL of methanol, add 8 g of sodium borohydride in batches at -15°C, and stir for 12 h. After the reaction is completed, add 15 g of citric acid, stir to dissolve, and add 10% dropwise. Concentration of sulfuric acid solution until the pH value of the solution is 3, and continue to stir the reaction for 1h. After the reaction was completed, 20% sodium hydroxide was added, the pH of the solution was adjusted to be alkaline, a white solid was precipitated, and filtered to obtain 16.5 g of erythromycin with a yield of 87% and a melting point of 114-116°C.
Embodiment 3
[0046] (1) Dissolve 15 g of azithromycin prepared by the method of Example 2 in methanol solution, stir and dissolve at -5 °C, add 4 mol / L sulfuric acid to adjust the pH value of the solution to 1-2, react for 6 h, and the reaction is completed Then, the pH of the solution was adjusted to 8-9 with 20% sodium hydroxide, extracted three times with an appropriate amount of dichloromethane, the filtrate was collected, and concentrated to obtain 7.2 g of crude TUM with a yield of 62%.
[0047] (2) Put 7.2g of TUM crude product in a three-necked flask equipped with a spherical condenser, add 100mL of a mixed solution of acetone and petroleum ether, the volume ratio of acetone and petroleum ether is 8:1, stir and dissolve at 60 ° C for 2h, then Cool to 0~5℃ for recrystallization for 6h, filter, and dry the obtained product. According to this method, it was recrystallized twice, and the obtained solid was dried in a vacuum drying oven at 50°C for 5 hours to obtain 3.9 g of pure TUM wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com