Transparent polyimide resin and preparation method thereof
A technology of polyimide resin and transparent polyimide, which is applied in the field of transparent polyimide resin and its preparation, can solve the problem that the transparency and heat resistance performance need to be further improved, it cannot be applied on a large scale, and it hinders the electron cloud. Conjugation and other issues to achieve the effects of inhibiting the formation of charge transfer complexes, large free volume, and increasing the glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
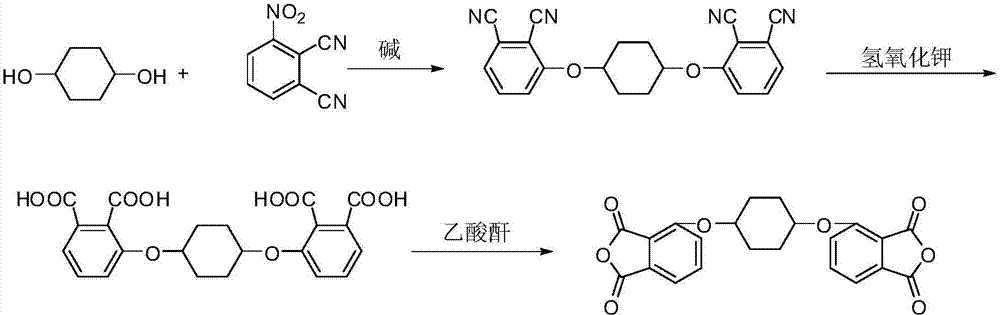
[0064] Embodiment 1: Preparation of 1,4-bis(2,3-dicarboxylic acid phenoxy)cyclohexane dianhydride:
[0065] Under nitrogen protection, add 8.4g (60% solid content, mass percentage, 210mmol) of sodium hydride into a 500mL three-necked flask, then add 100mL of N,N-dimethylformamide, and stir at room temperature for 2 hours. Then dropwise add a mixture of 11.6g (100mmol) of 1,4-cyclohexanediol and 100mL of N,N-dimethylformamide, after stirring at room temperature for 1 hour, add 35.5g (205mmol) The mixed solution of 3-nitrophthalonitrile and 150mL of N,N-dimethylformamide was stirred at room temperature for 3.5 hours after the addition was completed, the reaction solution was poured into water, a precipitate was precipitated, filtered, washed with ethanol, Drying gave crude 1,4-bis(2,3-dicyanophenoxy)cyclohexane.
[0066] The above crude product was heated to reflux in 150 mL of acetonitrile solution, filtered while hot, and the filter residue was dried to obtain 26.7 g of white...
Embodiment 2
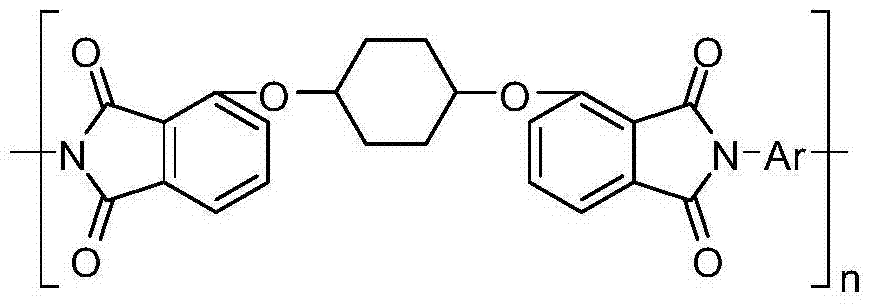
[0074] In this embodiment, the transparent polyimide resin material has the following structural formula:
[0075]
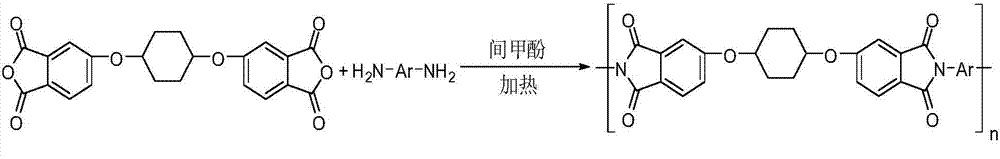
[0076] The specific preparation method is: under nitrogen protection, 1.3450g (4.0mmol) of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl ether, 1.6668g (4.0mmol) of 1, Add 4-bis(2,3-dicarboxylic acid phenoxy)cyclohexanedianhydride and 15ml of m-cresol into a 50mL reaction flask, then add 3 drops of isoquinoline, heat and stir at 190°C for 8 hours to obtain Viscous polyimide solution, when the reaction temperature drops to 60-80°C, dilute with m-cresol and precipitate in ethanol, after suction filtration, boil and wash twice with ethanol under reflux, put it in an oven to dry, and obtain polyimide imide product.
[0077] The inherent viscosity of the polyimide resin prepared in this example is 0.96dL / g, and the glass transition temperature is 245°C.
[0078] The polyimide powder prepared in this embodiment is configured into a solution with a concentration ...
Embodiment 3
[0086] In this embodiment, the transparent polyimide resin material has the following structural formula:
[0087]
[0088] The specific preparation method is: under nitrogen protection, 1.7300g (4.0mmol) of 4,4'-bis(3-aminophenoxy)diphenyl sulfone, 1.6668g (4.0mmol) of 1,4-bis(2 , 3-dicarboxylic acid phenoxy) cyclohexane dianhydride and 15 ml of m-cresol were added to a 50 mL reaction flask, and then 3 drops of isoquinoline were added, heated and stirred at 190°C for 8 hours to obtain a viscous polyamide The imine solution, when the reaction temperature drops to 60-80°C, is diluted with m-cresol and precipitated in ethanol. After suction filtration, the ethanol is refluxed and washed twice, and dried in an oven to obtain a polyimide product.
[0089] The inherent viscosity of the polyimide resin prepared in this embodiment is 0.34dL / g, and the glass transition temperature is 208°C.
[0090] The polyimide powder prepared in this embodiment is configured into a solution wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com