A method for preparing cytokine and bone protein microspheres by thin film evaporation
A technology of cytokines and morphogenic proteins, which is applied in the field of biomedicine, can solve the problems of low bioavailability, high protein loss, and difficult measurement of microglobulin encapsulation rate, and achieve simple and stable preparation methods, uniform volume, and improved The effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] [Example 1] Optimization of membrane composition
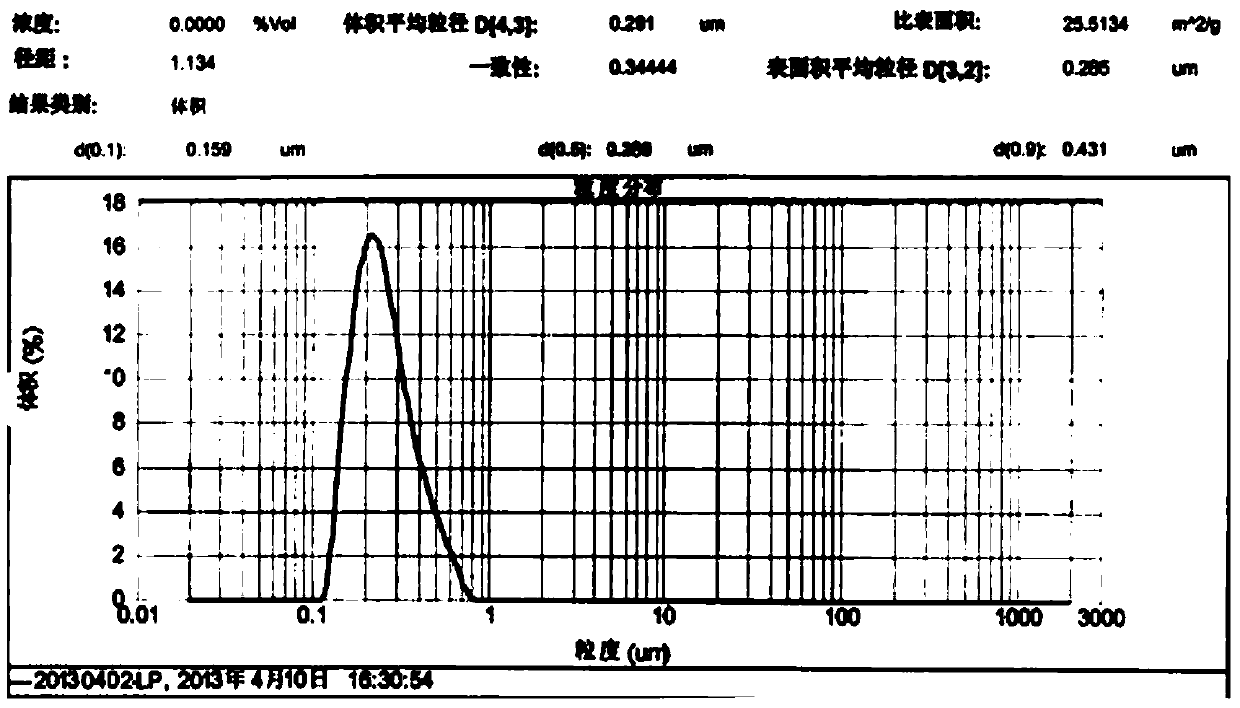
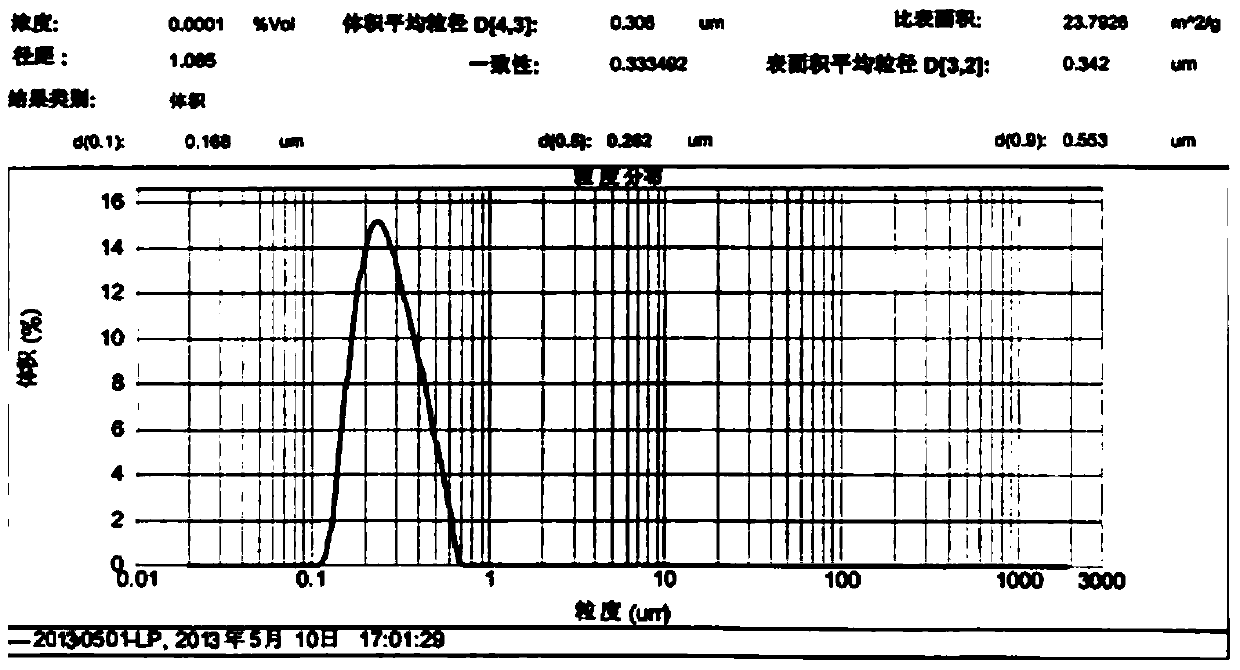
[0043] Orthogonal test design without considering the interaction was used to determine the optimal formulation of lipid microsphere membrane materials, and the best composition of three components including lecithin, cholesterol and octadecylamine for injection in lipid microsphere membrane materials was selected. proportional formula. Its preparation method is to adopt the optimized thin film evaporation method, and use an ultra-high pressure homogenizer for uniform crushing, and then conduct particle size analysis, encapsulation efficiency, stability and other indicators related to lipid microspheres, and set the parameters according to each individual indicator. There are 5 grades from poor to excellent, according to the grades (1-5, 5 points), and finally the best formula is obtained according to the analysis of variance.
[0044] (1) The selection results of the best membrane materials are shown in Table 1
[00...
Embodiment 2
[0050] [embodiment 2] the selection of solvent
[0051] The effects of four different solvents on the prepared lipid microspheres were compared, and the results are shown in Table 2:
[0052] Table 2: Comparison of the effects of four different solvents on the prepared lipid microspheres (0 month)
[0053]
[0054] Note: The smaller the oxidation index value, the smaller the degree of oxidation of phospholipids
[0055] ★★ The smaller the diameter value, the particle size tends to be normally distributed, and the lipid microsphere particles are more uniform
[0056] See Table 3 for the influence of four different solvents on the particle size stability of the prepared lipid microspheres:
[0057] Table 3: Effects of four different solvents on the particle size stability of the prepared lipid microspheres
[0058]
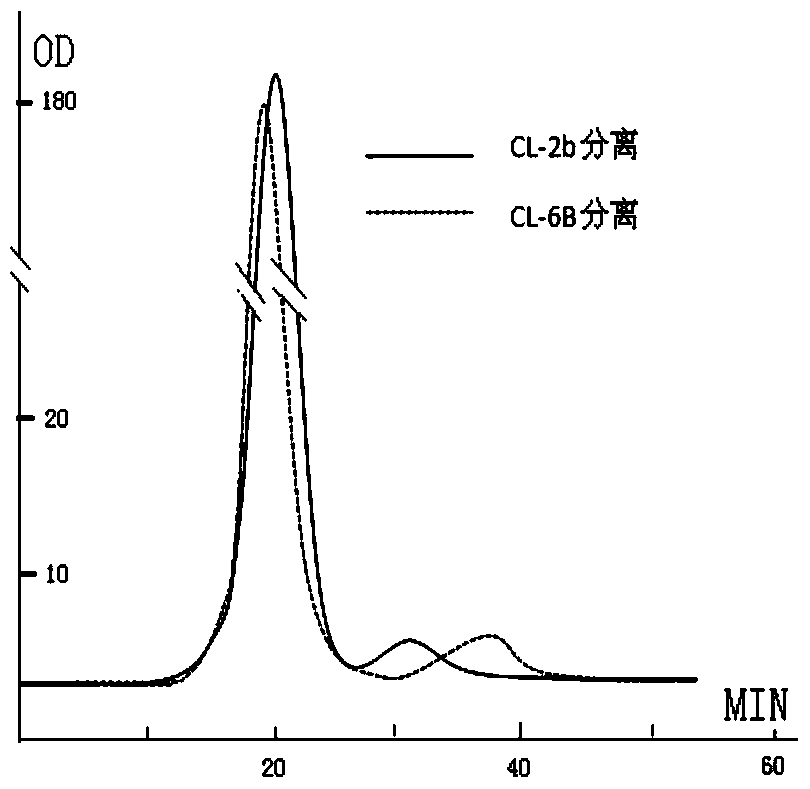
[0059] The solvent of the membrane material is an important factor affecting the morphology and encapsulation efficiency of lipid microspheres during the ...
Embodiment 3
[0061] [Example 3] Preparation of Lipid Microspheres
[0062] The steps of preparing lipid microspheres by thin film evaporation method are as follows: ①Film formation: membrane material ingredients: lecithin for injection, cholesterol and octadecylamine (or vitamin E) are weighed according to the ingredient ratio of 3-7:1-3:1, Then dissolve it in dichloromethane or trichloromethane with a final concentration of 0.5-1.5%, put the liquid in an eggplant-shaped bottle of a rotary evaporator, use a water bath at 60°C as the evaporation temperature, rotate to evaporate the solvent, and use nitrogen gas in the final stage. Dry the solvent to make the liposomes form a film in an eggplant-shaped bottle. ②Hydration: Add about 50ml of the aqueous phase solution of cytokine recombinant human IFNβ1a (final concentration: 100ng / ml) and bone morphogenetic protein BMP-2 (final concentration: 80ng / ml) (the concentration of the membrane material in the solution is 1% ), add a few small glass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com