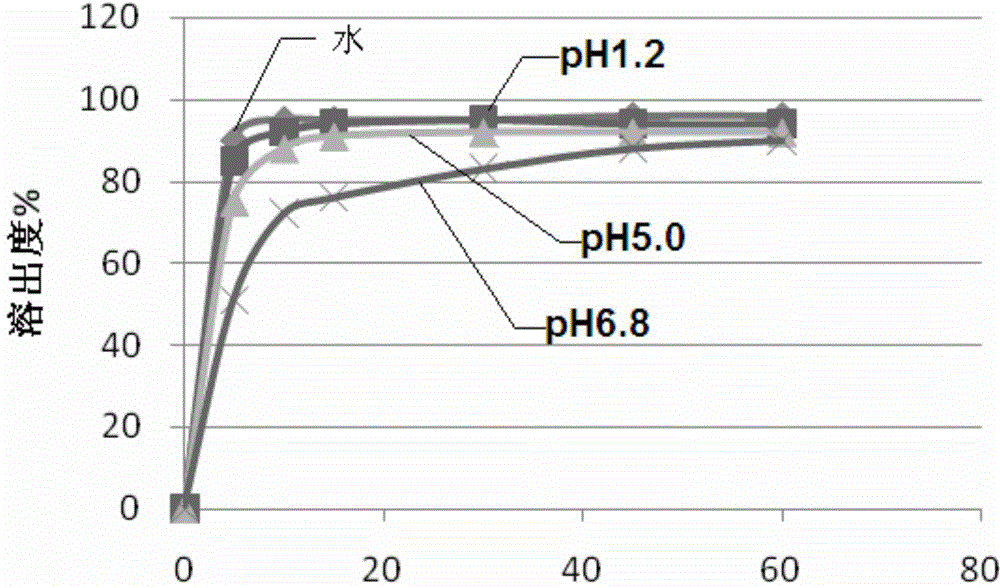
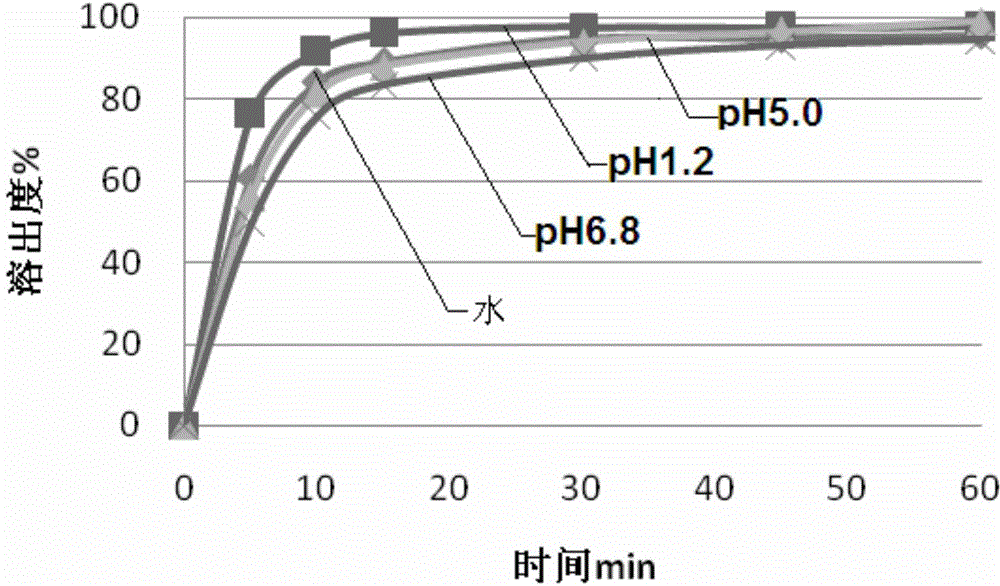
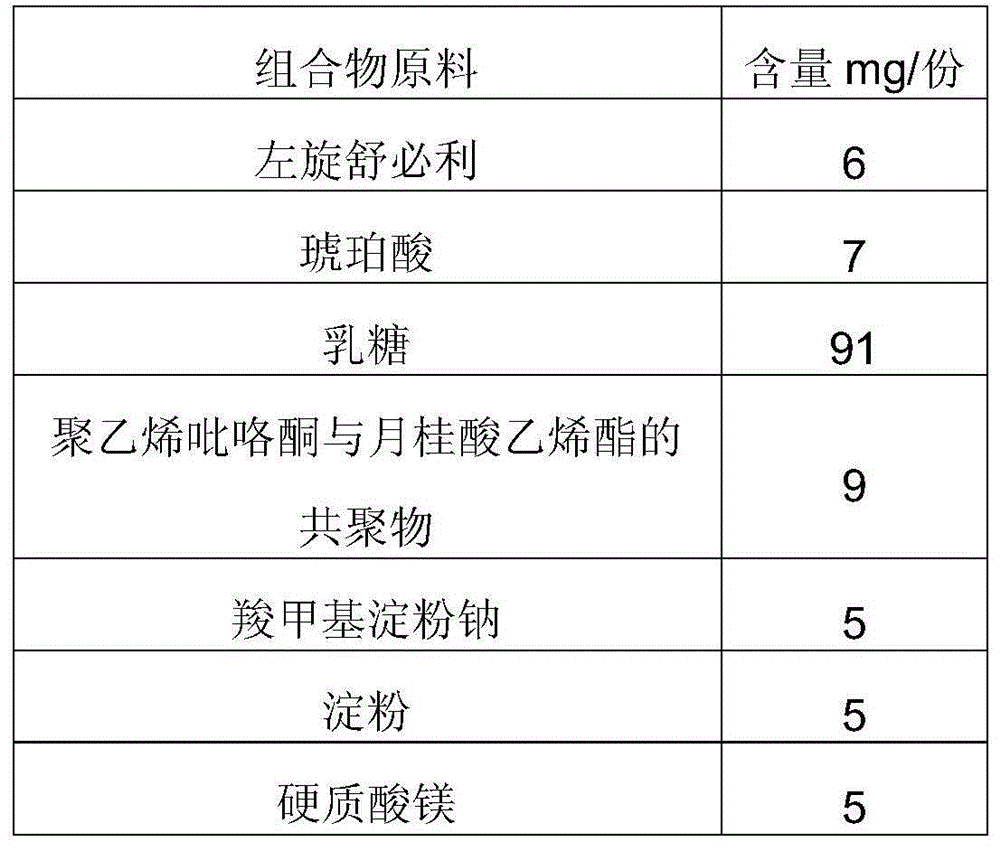
Composition for improving dissolution rate of benzamide gastric motility drug and application of composition
A technology of benzamides and gastric motility, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor dissolution, achieve high dissolution, improve curative effect, and good The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Prepare a composition capsule that improves the dissolution rate of benzamide gastric motility drugs, specifically capsules containing mosapride citrate, the specific amount of raw materials of the composition is as shown in Table 1:
[0055] Table 1, the composition raw material list of preparation mosapride citrate capsule
[0056] composition raw material
Content mg / grain
5.3
90.2
10
Hydroxypropyl methylcellulose RT5 (HPMC RT5)
8
8
Micropowder silica gel
0.5
[0057] The preparation steps are as follows:
[0058] Pass the mosapride citrate bulk drug through a 200-mesh sieve, and other excipients through a 80-mesh sieve;
[0059] Dissolve 5 mg of hydroxypropyl methylcellulose in 100 ml of purified water, add the raw material drug into the solution, put the rest of the materials into a fluidized bed granulator and mix for 3 minutes...
Embodiment 2
[0062] Prepare a composition tablet that improves the dissolution rate of benzamide gastric motility drugs, specifically a tablet containing cisapride, and the specific consumption of the raw materials of the composition is as shown in Table 2:
[0063] Table 2, the composition raw material list of preparation cisapride tablet
[0064] composition raw material
Content mg / tablet
Cisapride
6
8
[0065] Cyclodextrin
92
Hydroxypropylmethylcellulose
8
10
3
Micro silica gel
4
[0066] The preparation steps are as follows:
[0067] The above-mentioned components are uniformly mixed, and each component is compressed by a general wet granule compression method, and the tablet obtained by compression is coated with a protective film to obtain the desired tablet containing cisapride.
Embodiment 3
[0069] Prepare a composition tablet that improves the dissolution rate of benzamide gastric motility drugs, specifically a tablet containing metoclopramide, and the specific amount of raw materials of the composition is as shown in Table 3:
[0070] Table 3, the composition raw material list of preparation metoclopramide tablet
[0071] composition raw material
Content mg / tablet
6
5
3
92
Copolymer of polyvinylpyrrolidone and vinyl chloride
8
10
3
Micro silica gel
4
[0072] The preparation steps are as follows:
[0073] The above-mentioned ingredients are uniformly mixed, and the ingredients are compressed and manufactured by the general direct powder compression method, and the tablets obtained by the compression manufacturing are coated with a slow-release film t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com