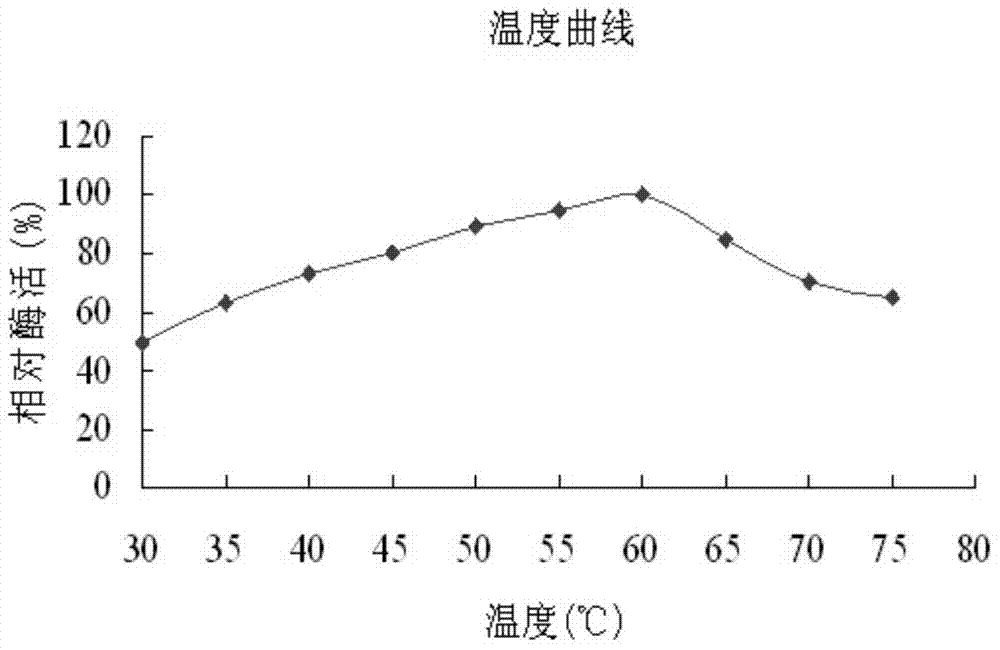
Alpha-glucosidase and application of alpha-glucosidase
A technology of glucosidase and isomaltose, applied in the fields of application, glycosylase, enzyme, etc., can solve the problems of low enzyme expression and high cost, and achieve the effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Cloning of α-glucosidase gene
[0020] Genomic DNA was extracted from S. niger overnight cultures using a fungal genomic DNA extraction kit (Omega).
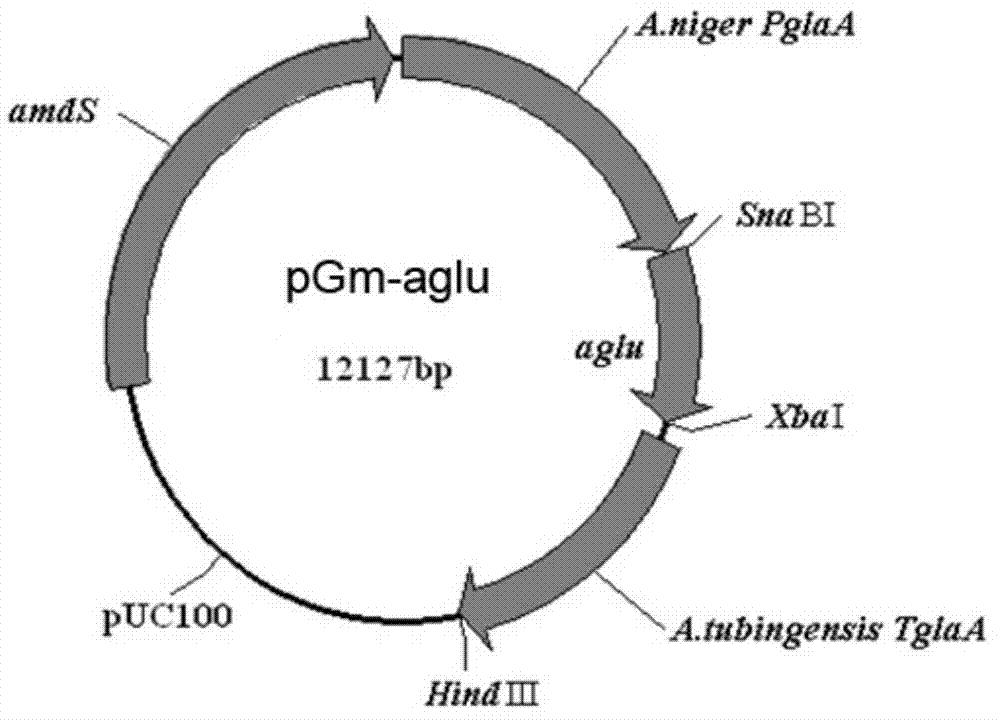
[0021] Amplify the α-glucosidase gene in Staphylococcus niger with the genomic DNA of Staphylococcus niger as the amplification template, and the forward primer aGluS6480-F sequence used is (the sequence shown in the underline is the SnaBI restriction site) ;5′-CCAT TACGTA AGA ATGAGACTTGGGCTCGCCATCCTG-3′,
[0022] The sequence of the reverse primer aGluS6480-R is (the underlined sequence is the XbaI restriction site):
[0023] 5′-TGC TCTAGA TTAGAGCTCGTCCGAGCCCAACGCTTCCT - 3′
[0024] The gene was amplified from S. niger genomic DNA using Phusion DNA polymerase (Thermo scientific).
[0025] The above PCR products were purified using a gel purification kit (Fermentas). The purified PCR product was digested with restriction enzymes SnaBI and XbaI (Fermentas); at the same time, the plasmid pGm was digeste...
Embodiment 2
[0028] Example 2: PEG-mediated protoplast fusion transformation and verification of Aspergillus niger
[0029] Pipette the spore suspension of the host bacteria Aspergillus niger G1 (Aspergillus niger G1) in the center of the CMA plate (9cm petri dish), when the colony covers the entire petri dish, cut 1 / 4 of the size of the culture based on 200mL CMA liquid medium, in 30 Cultivate at 200rpm for 14-16h.
[0030] Collect the mycelium with sterile Miracloth filter cloth, wash it once with solution A, transfer the washed mycelium to 40 mL of protoplastization solution under aseptic conditions, and warm it at 30 °C and 200 rpm for 1 to 2 h. Protoplast transformation progress was detected by microscope observation.
[0031] Filter the above warm bath liquid with sterile Miracloth filter cloth, and the obtained filtrate is the protoplast solution. The protoplast solution was divided into two 50 mL sterile disposable centrifuge tubes, and the volume of each tube was made up to 45 m...
Embodiment 3
[0044] Example 3: Fermentation of Aspergillus niger aglu and expression of α-glucosidase
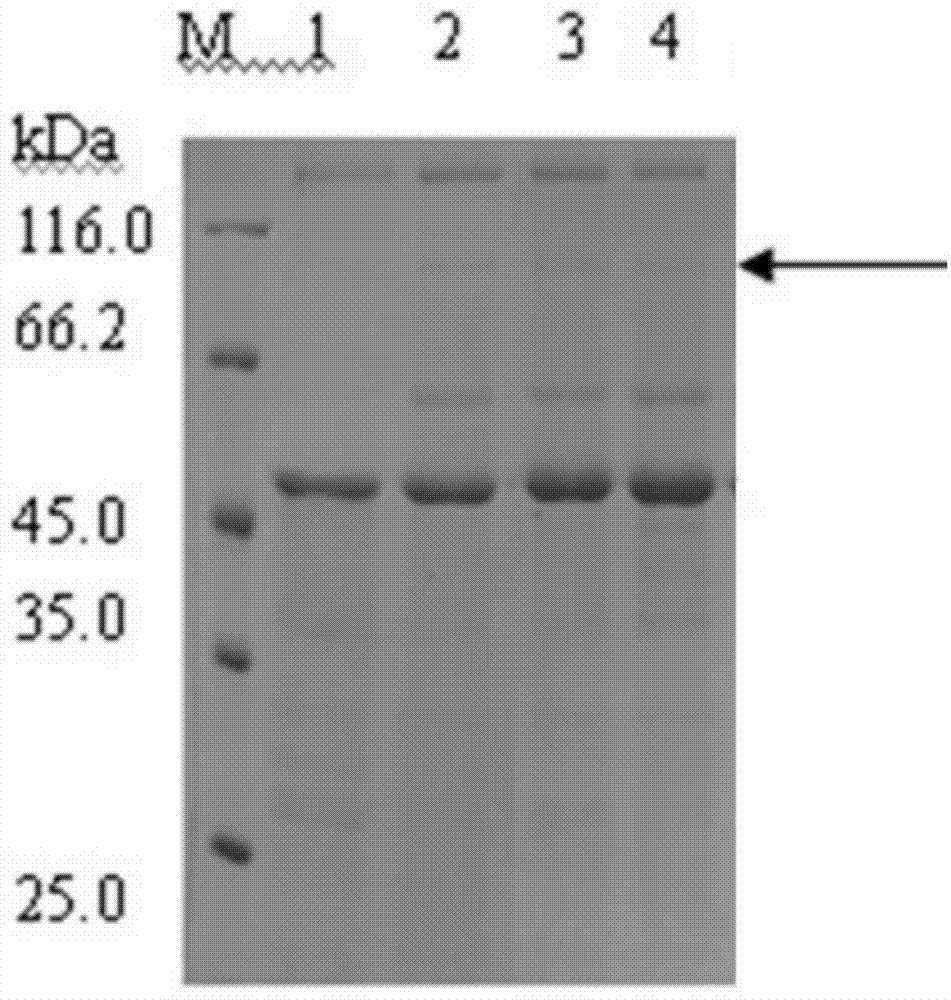
[0045] Pick the positive transformant Aspergillus niger aglu obtained in Example 2, inoculate it in 30 mL of TSB fermentation medium, and cultivate for 5 d at 30° C. and 200 rpm; at the same time, take the host bacterium Aspergillus niger G1 not transformed into the exogenous gene as The control group was cultivated under the same fermentation conditions as above; the fermentation broths of the obtained Aspergillus niger aglu and host bacteria Aspergillus niger G1 were filtered with 8 layers of gauze respectively, and the filtrate was centrifuged for 10 min under 14000g conditions, and the supernatant was collected; the above-mentioned supernatant was Electrophoresis was performed on a 12% SDS-PAGE gel.
[0046] The result is as figure 2 Swimming lane 1 shows the expression of the secreted protein of the host strain Aspergillus niger G1, and lanes 2-4 show the expression of the secrete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com