Itraconazole inhalation powder spray and preparation method thereof
A technology for inhaling powder and itraconazole, which is applied in the field of medicine, can solve the problems of significant individual differences, unstable absorption, and large food influence, and achieve the effects of less dosage, good physical stability, and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
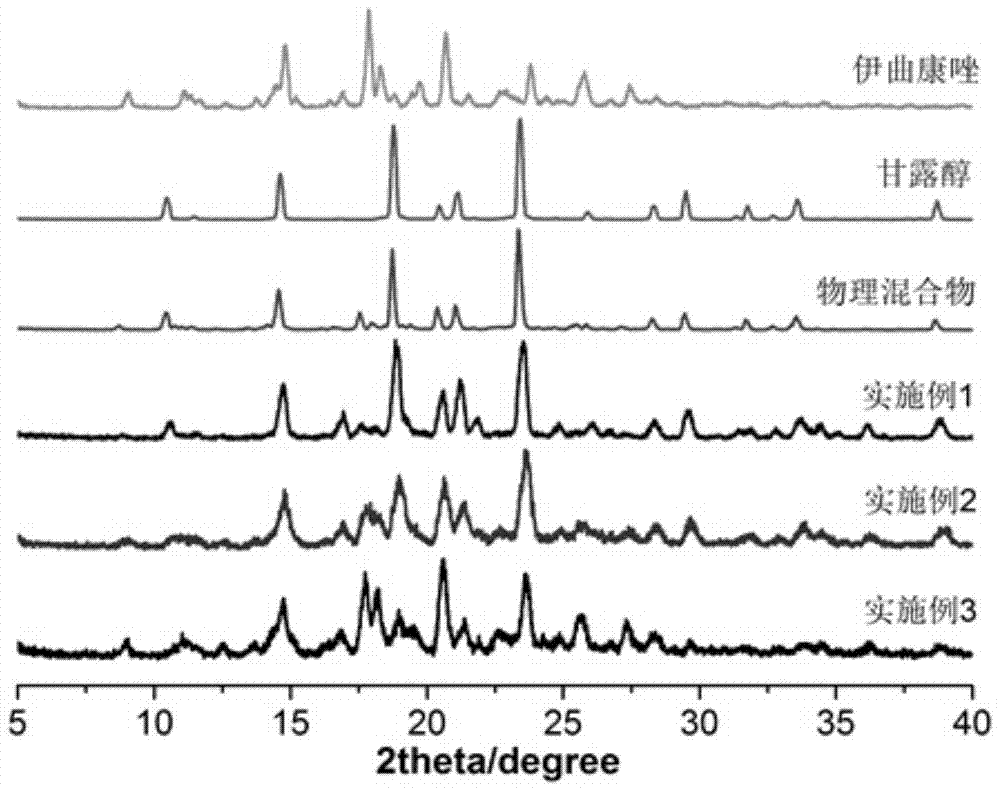
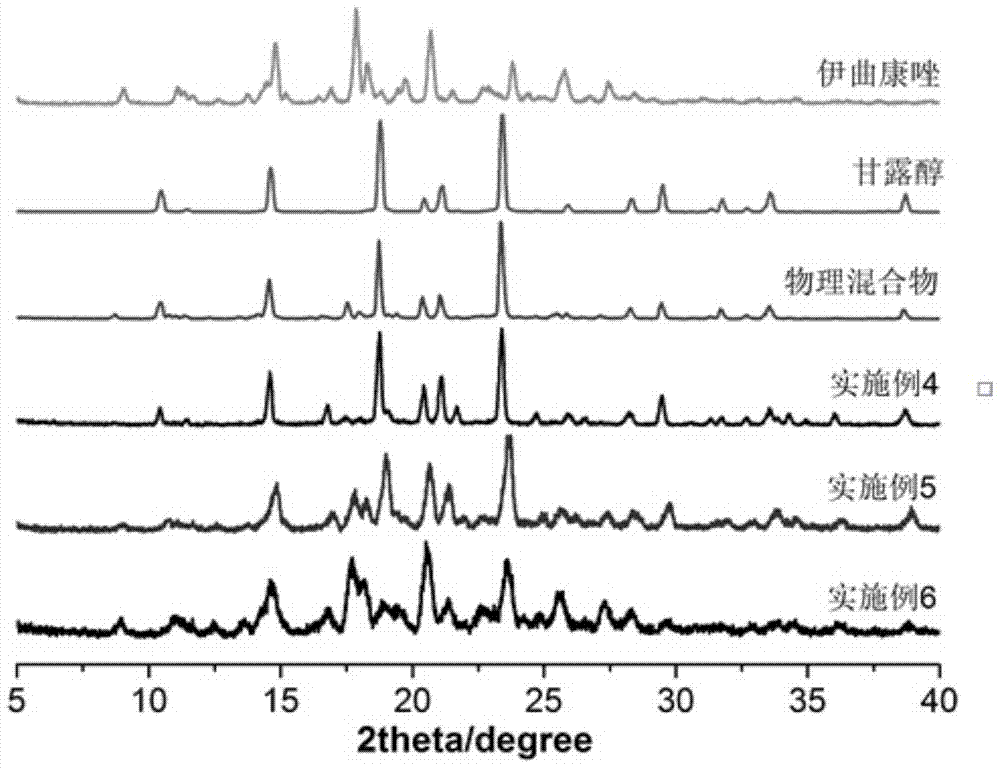
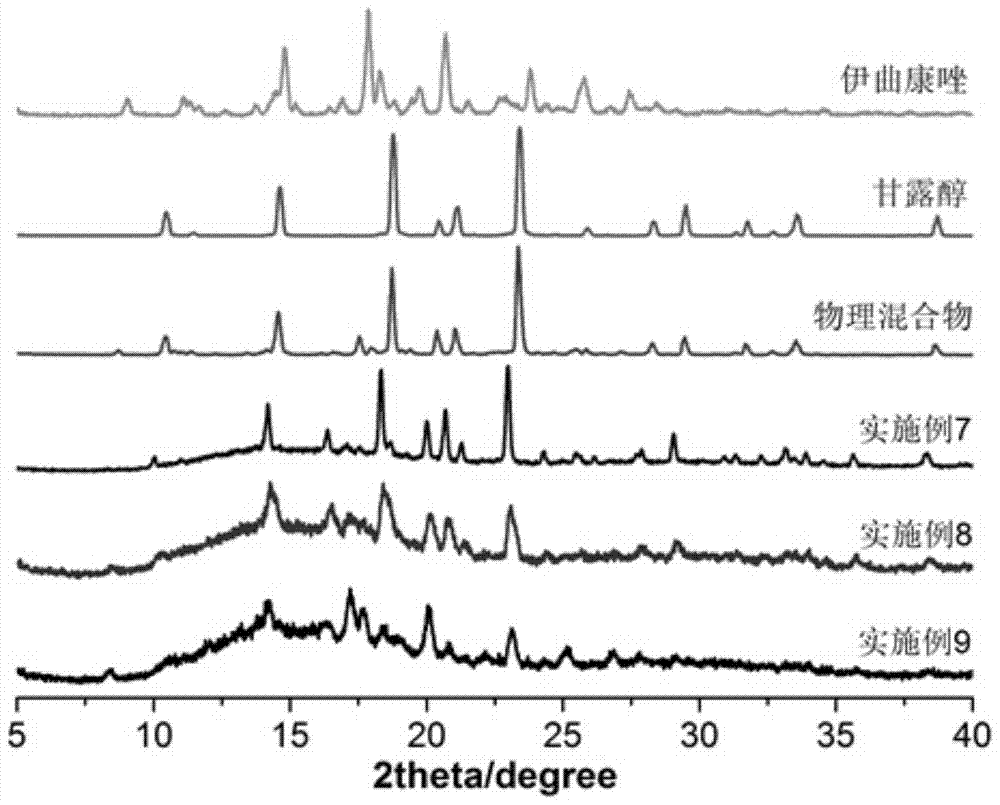
Embodiment 1
[0041] In this example, an itraconazole inhalation powder is prepared from 2.002 g of itraconazole as the active ingredient and 7.998 g of the carrier mannitol.
[0042] The preparation method of above-mentioned itraconazole inhalation powder and spray, comprises the steps:
[0043] (1) Get 2.002g itraconazole crude drug, 7.998g mannitol, mix homogeneously, obtain mixture;
[0044] (2) Set the extrusion temperature of the twin-screw hot-melt extruder to 160°C, start the screw after reaching the preset temperature, the screw speed is 150 rpm, and add the uniformly mixed physical mixture into the extrusion by manual injection In the machine, the mixture is extruded in strips through the screw; the hot-melt extruded strips are placed in a drier to cool at room temperature, and the itraconazole solid dispersion is obtained by preliminarily pulverizing with a micro-powder machine, and the obtained itraconazole The solid dispersion is then passed through a jet mill (nozzle air pres...
Embodiment 2
[0047] This example is an itraconazole inhalation powder, the components of which are basically the same as those in Example 1, the difference is that the raw material of itraconazole is 4.996g, and that of mannitol is 5.004g.
Embodiment 3
[0049] This example is an itraconazole inhalation powder, the components of which are basically the same as those in Example 1, the difference is that the raw material of itraconazole is 7.982g, and that of mannitol is 2.018g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com