PHBV (polyhydroxylbutyrate valerate) polymer, preparation method and hemostatic material manufactured by using PHBV polymer
A polymer and copolymer technology, applied in the biological field, can solve the problems of increasing the chance of wound infection, endangering life, and decreasing hardness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
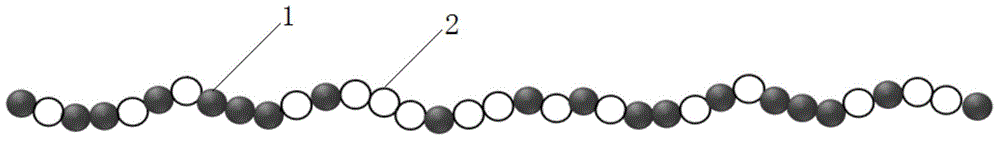
[0029]This embodiment provides a PHBV polymer, the polymer is a copolymer of 3-hydroxybutyrate 3HB and 3-hydroxyvaleric acid 3HV, and the main chain of the polymer includes at least fifty poly 3-hydroxy A block of butyric acid and a block of at least fifty poly-3-hydroxyvaleric acid.
[0030] The main chain of the above-mentioned polymer also includes random copolymerization segments of 3-hydroxybutyric acid and 3-hydroxyvaleric acid;
[0031] The molar ratio of comonomers 3HB and 3HV in the polymer is 20-50:80-50, preferably 33-50:67-50;
[0032] The weight average molecular weight of the polymer is 1~3×10 6 , the molecular weight polydispersity coefficient is 1.3-2.2, preferably 1.5-1.8.
[0033] The thermodynamic parameters of the above-mentioned PHBV polymer are as follows: glass transition temperature-15~5℃, preferably-10~3℃; cold crystallization temperature 55~72℃, preferably-62~70℃; melting temperature 132-145℃, Preferably 135-145°C; decomposition temperature 260-275...
Embodiment 2
[0054] This embodiment provides the microstructure analysis of embodiment 1 PHBV
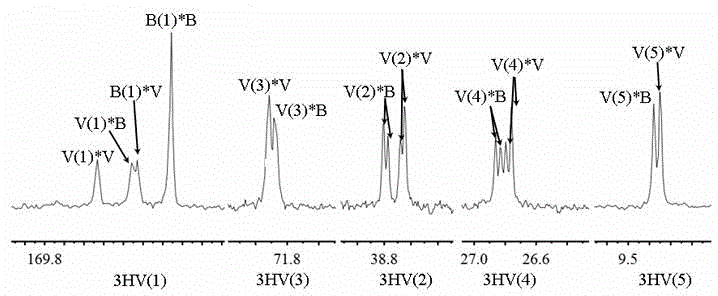
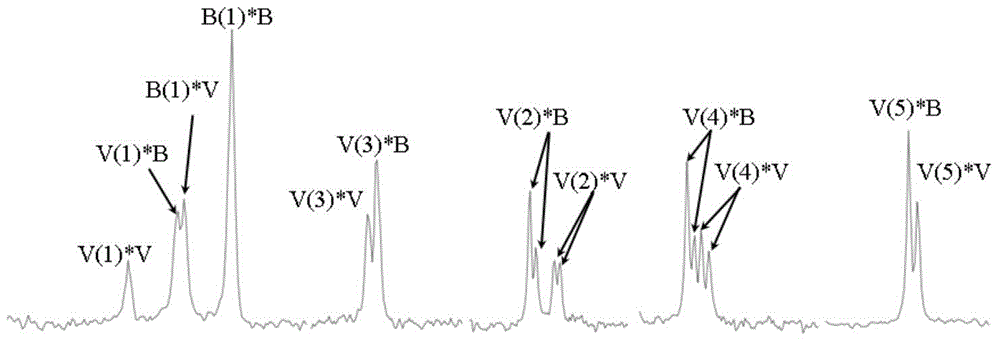
[0055] The purified PHBV sample was dissolved in deuterated chloroform at a concentration of 20 mg / mL, and methylsilane was used as an internal standard. The samples were analyzed by nuclear magnetic resonance (Bruker AVANCE III 500, Germany) at room temperature. 13 Data acquisition for C NMR. The sample recorded a spectral width of 10,000 Hz on the spectrometer, and 32k data points were accumulated 30,000 times 13 C NMR.
[0056] Figure 1A with 1B It is the PHBV microstructure analysis figure provided by the present embodiment, in the figure is 13 3HV NMR spin-splitting peak in the C NMR spectrum. Among them, the 3HV molar content in the random copolymerized PHBV sample is 30.1%, and the 3HV molar content in the highly regular copolymerized PHBV sample is 33.3%, that is, the sample with a special microstructure obtained in this embodiment; the 3HV monomer content of the two is basically t...
Embodiment 3
[0072] This embodiment analyzes the molecular weight and its distribution of the PHBV material, and its thermodynamic properties
[0073] The molecular weight of the above-mentioned highly regular polymer PHBV was determined by gel retardation chromatography (Agilent HPLC 1260, USA). Chloroform was used as the mobile phase at a flow rate of 1.0ml / min. Polystyrene (1.01×10 4 ,2.88×10 4 ,7.48×10 4 ,2.15×10 5 ,5.08×10 5 ,9.91×10 5 , and 3.04×10 6 ) (Agilent, USA) was used as the standard molecular weight to make a standard curve. The results show that the weight-average molecular weight of the highly regular polymer PHBV is 2.0×10 6 , Molecular weight polydispersity coefficient 1.63.
[0074] The thermodynamic analysis of the above highly regular polymer PHBV was carried out using a differential calorimetry scanner (DSC Q2000, USA) and a thermogravimetric analyzer (TGA Q500, USA). The measurement results are as follows: the glass transition temperature is -8.91°C and -0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Cold crystallization temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com