A dual-functional negative electrode and its application as negative electrode for all-vanadium redox flow battery
An all-vanadium redox flow battery, dual-function technology, applied in battery electrodes, fuel cells, regenerative fuel cells, etc., can solve the problems of high electrode cost, reduced hydrogen evolution, unsuitable for large-scale application, etc. Effects of hydrogen evolution, enhanced electrocatalytic activity and electrochemical reversibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A certain size of carbon felt was impregnated in 0.01M Bi(NO 3 ) 3 HNO 3 solution, after ultrasonic dispersion for 30 min, take it out, put it in a drying oven at 105 ° C for 10 h, and then place the loaded Bi(NO 3 ) 3 The carbon felt was heated to 600°C in a nitrogen atmosphere, and H 2 Constant temperature reaction 1h, the Bi 3+ It was reduced to Bi, then cooled to room temperature under a nitrogen atmosphere, and weighed using an electronic balance to determine that the mass ratio of Bi loading was 1%.
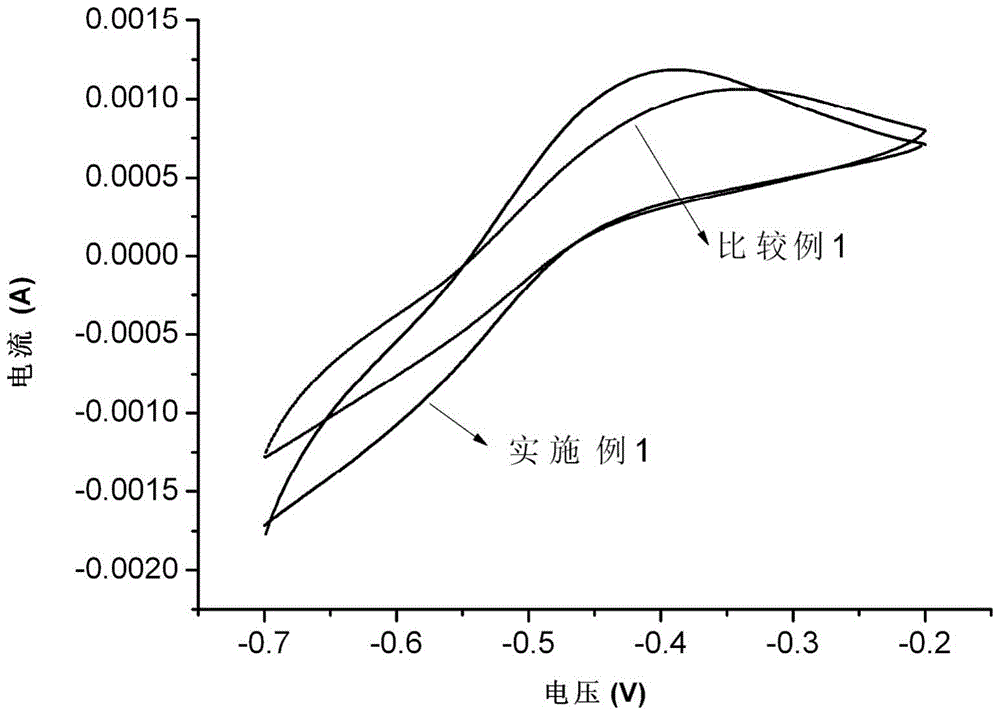
[0053] In order to test the electrochemical activity of vanadium ion redox couple on the surface of Bi-modified carbon felt, the Bi-modified carbon felt prepared in Example 1 was tested by cyclic voltammetry. Bi-modified carbon felt was used as the working electrode, a non-porous graphite plate was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode. The electrochemical testing instrument used was the CHI612 elect...
Embodiment 2
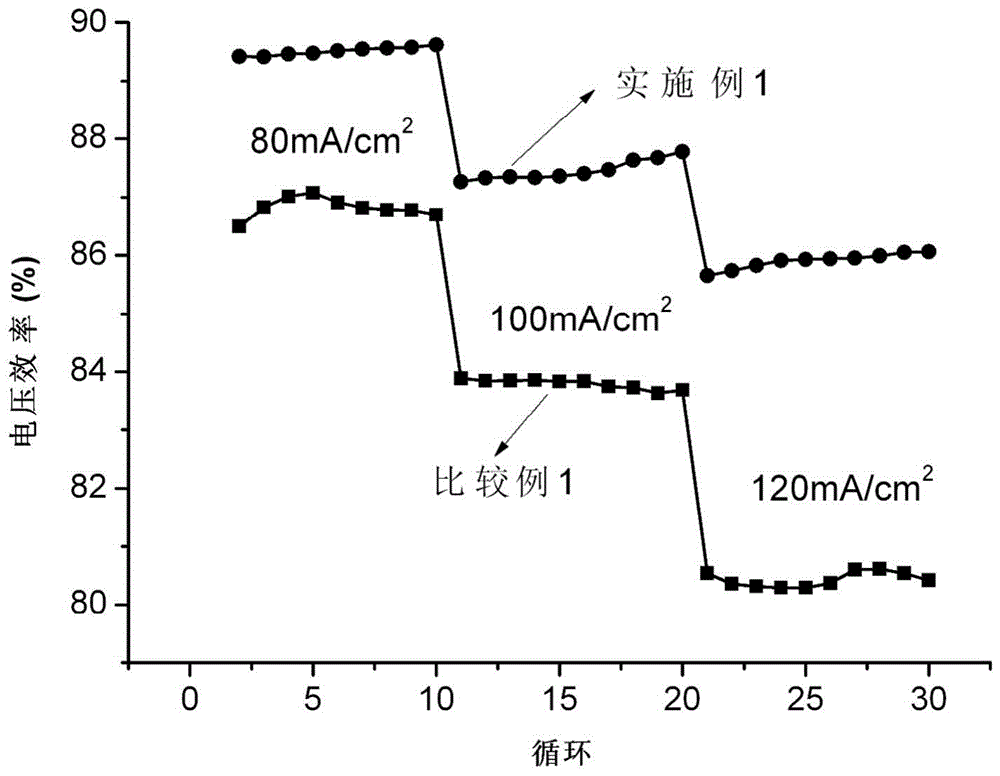
[0083] The electrodeposition solution consists of 12g / L BiCl 3 , 55g / L tartaric acid, 100g / L glycerin and 45g / L sodium chloride solution, the pH value of the solution is adjusted to about 1.0 with dilute hydrochloric acid. A carbon felt of a certain size is used as the working electrode, and the counter electrode is a graphite plate. Direct current electrochemical deposition is adopted, and the current density is 10mA / cm 2 , the deposition time is 10s. The mass ratio of Bi loading was determined to be 1% by weighing with an electronic balance. The negative electrode material not only has high electrocatalytic activity, can reduce the electrochemical polarization of the liquid flow energy storage battery, and increase the working current density of the battery; it also has a high hydrogen evolution overpotential, which can inhibit hydrogen evolution and improve the battery at high operating current density. the next lifespan.
Embodiment 3
[0086] A certain size of graphite felt was impregnated in 0.02M Bi(NO 3 ) 3 in the ethylene glycol solution, ultrasonically dispersed for 30min, took it out, put it in a drying oven at 200°C for 10h, and then loaded the Bi(NO 3 ) 3 The graphite felt is heated up to 500°C in a nitrogen atmosphere, and H 2 Constant temperature reaction 2h, Bi 3+ It was reduced to Bi, then cooled to room temperature under a nitrogen atmosphere, and weighed using an electronic balance to determine that the mass ratio of Bi loading was 2%. The negative electrode material not only has high electrocatalytic activity, can reduce the electrochemical polarization of the liquid flow energy storage battery, and increase the working current density of the battery; it also has a high hydrogen evolution overpotential, which can inhibit hydrogen evolution and improve the battery at high operating current density. the next lifespan.
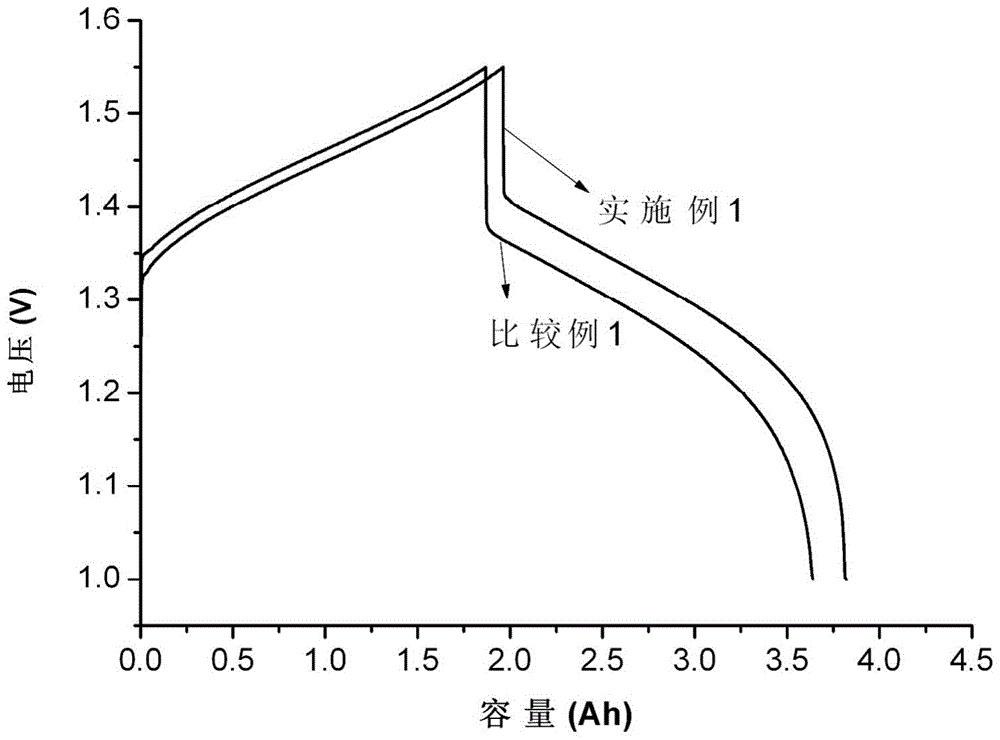
[0087] The single cell assembly evaluation conditions are the same as i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com