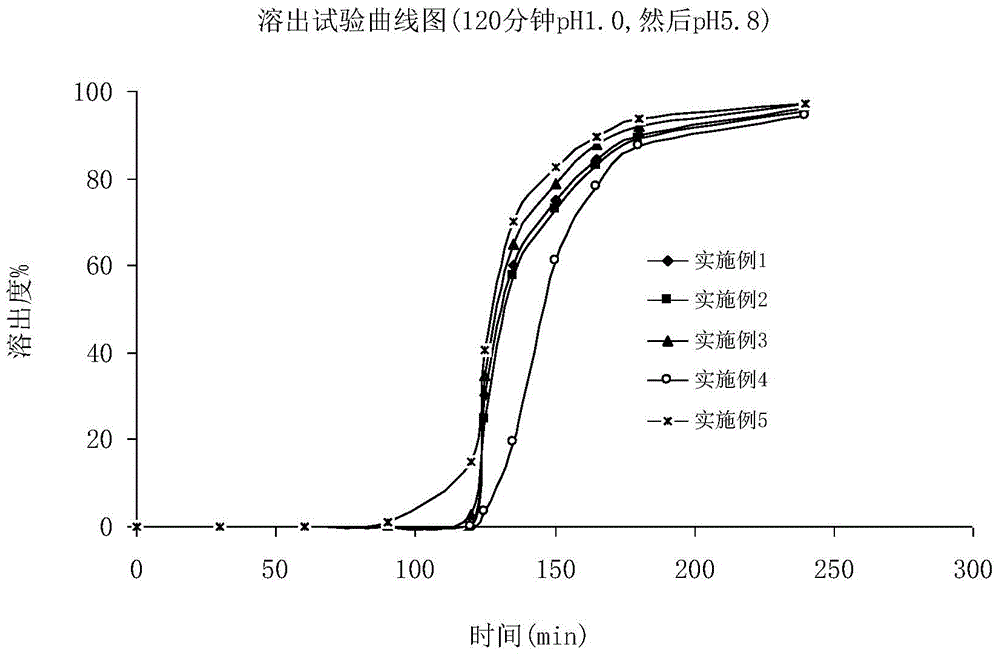
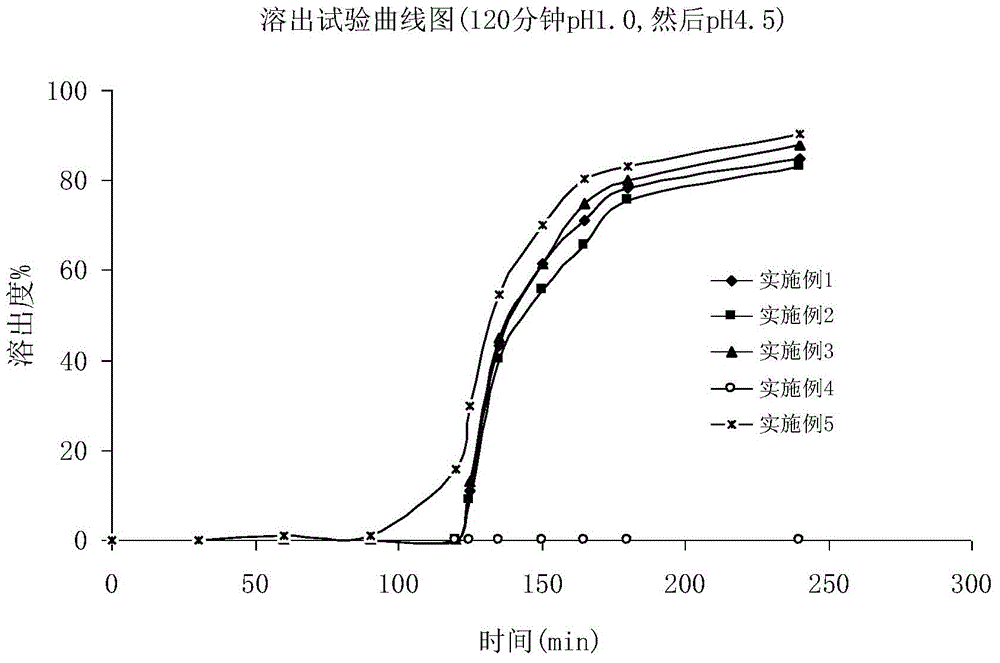
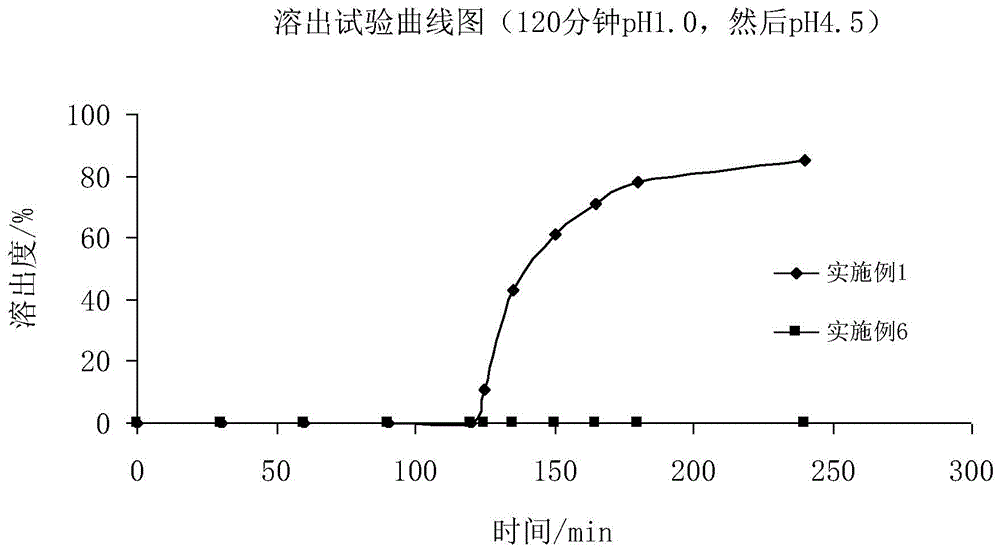
Fidaxomicin enteric-coated preparation
A fidaxomicin enteric and preparation technology, which is applied in the field of western medicine preparations, can solve the problems of hidden dangers in the use of fidaxomicin, slow drug onset, and difficult-to-dissolve films, so as to improve bioavailability, improve bactericidal efficacy, and prepare The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 The product of this example is Fidaxomicin enteric-coated tablets.
[0048] (1) chip core
[0049] prescription:
[0050]
[0051]
[0052] Preparation: Mix the prescribed amount of Fidaxomycin with microcrystalline cellulose, starch, hypromellose, croscarmellose sodium, and magnesium stearate, pass through a 20-mesh sieve with water-made soft materials, and prepare Granules; dried at 50°C to 60°C, passed through a 16-mesh sieve for granulation. Measure the content of the granules and press into tablets to obtain the fidaxomicin tablet core.
[0053] The obtained fidaxomicin tablet cores each weigh 250 mg and contain 140 mg of fidaxomicin.
[0054] (2) isolation gown layer
[0055] prescription:
[0056]
[0057] Dosing solution: hypromellose E 5 Slowly add water, after fully dispersed, add polyethylene glycol and polyvinyl alcohol copolymer, talcum powder, stir evenly to obtain the isolation coating solution. The solid content of the isolation...
Embodiment 2
[0066] The product in this example is Fidaxomicin enteric-coated pellets.
[0067] (1) ball core
[0068] prescription:
[0069]
[0070] Preparation: Mix the prescribed amount of fidaxomicin, microcrystalline cellulose, starch, hydroxypropyl methylcellulose, and cross-linked carmellose sodium), and use hydroxypropylmethylcellulose dispersion as a binder The soft material is prepared by the agent, and the ball core is obtained by extruding and spheronizing. The obtained fidaxomicin ball core has a diameter of 0.5 mm to 0.6 mm.
[0071] (2) isolation gown layer
[0072] prescription:
[0073]
[0074]
[0075] The dosing method and the coating method are the same as in Example 1. The difference is that the rotating speed of the coating pan during the coating process is 50 rpm, and the coating is carried out by gap spraying. After coating, the weight of the isolation coat layer increased by 2.0%.
[0076] (3) Casing layer
[0077] prescription:
[0078]
[0...
Embodiment 3
[0082] The product in this example is Fidaxomicin enteric-coated granules.
[0083] (1) Inner core particles
[0084] prescription:
[0085]
[0086] Preparation: pass fidaxomicin, microcrystalline cellulose, starch and sodium carboxymethyl starch through a 40-mesh sieve, place in a rotary granulator, use hydroxypropyl cellulose aqueous solution as a binder, granulate, and sieve Select 16-30 mesh drug-containing granules and dry them at 40°C.
[0087] (2) isolation gown layer
[0088] prescription:
[0089]
[0090] The dosing method and the coating method are the same as in Example 1. The difference is that: the solid content of the isolation coat coating liquid is about 18.0%; the coating equipment is a rotary granulator. The weight gain of the gown layer was 2.0%.
[0091] (3) Casing layer
[0092] prescription:
[0093]
[0094] The solution preparation method and the coating method are the same as in Example 1, and the weight of the enteric coating layer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com