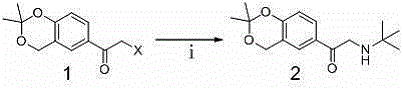
The synthetic method of levalbuterol intermediate and levalbuterol hydrochloride
A technology of levalbuterol and hydrochloride, which is applied in the field of preparation of levalbuterol intermediate and levalbuterol hydrochloride, can solve the problems of high production cost, many synthesis steps, low product yield and the like, and achieves short synthetic route and high yield. The effect of high rate and simple post-processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of embodiment one bromoacetyl salicylaldehyde
[0042] .
[0043]Add anhydrous aluminum trichloride (20 g, 0.15 mol) to a 250 mL three-necked flask, add 15 mL of dichloromethane dropwise under stirring, raise the temperature to 50°C, add dropwise 7 g (0.045 mol) of dichloromethane bromoacetyl chloride Methane solution 10 mL, stirred for 30 minutes. Dissolve 3.66 g (0.03 mol) of salicylaldehyde in 10 mL of dichloromethane, drop into the reactant at 40°C, and react under reflux for 12 hours. Slowly pour the reaction solution into 120 g of crushed ice under stirring, adjust the pH value to 4, stir for 30 minutes, then add dichloromethane, separate the organic layer, and wash the aqueous layer with dichloromethane (30 mL×3) , combined the organic phases, and the organic phases were washed with distilled water and saturated brine successively, then dried with anhydrous magnesium sulfate, filtered, concentrated, and vacuum-dried to obtain a purple-black oil,...
Embodiment 2
[0045] Example 2 2-bromo-1-(4-hydroxyl-3-(hydroxymethyl)phenyl)ethanone
[0046] .
[0047] Dissolve 12 g of bromoacetyl salicylaldehyde in 50 mL of acetic acid, then cool to 0 °C, add 2.3 g of sodium borohydride in batches under nitrogen protection, slowly warm up to room temperature after the addition, and continue stirring until the reaction is complete. Adjust the pH value of the reaction solution to neutral with saturated sodium carbonate aqueous solution at 0°C, then add 100 mL of distilled water, back-extract the mixed solution with ethyl acetate three times, combine the organic phases, and use distilled water and saturated salt for the organic phases in turn Wash with water, then dry with anhydrous sodium sulfate, filter, concentrate, and dry in vacuo to obtain the crude product, which is a red oily substance. g 2-bromo-1-(4-hydroxy-3-(hydroxymethyl)phenyl)ethanone, the property is light yellow solid, the yield is 88%.
[0048] 1 H-NMR ( DMSO-d 6 ): 4.36 (s, 2H),...
Embodiment 3
[0049] Example 3 Synthesis of 2-bromo-1-(2,2-dimethyl-4H-benzo[1,3]dioxin-6-yl)ethanone
[0050] .
[0051] 10 g of 2-bromo-1-(4-hydroxyl-3-(hydroxymethyl)phenyl)ethanone prepared in Example 2 and 0.14 g of p-toluenesulfonic acid in a catalytic amount were added to a 250 mL three-port In the flask, then add 100 mL of dichloromethane and stir to form a light yellow suspension. At room temperature, the solution formed by 8.5 g of 2,2-dimethoxypropane and 40 mL of dichloromethane is slowly added dropwise to the reaction solution. Continue to stir the reaction until the reaction solution becomes clear, add saturated potassium bicarbonate solution to the reaction solution to adjust the reaction system to weak alkalinity, let stand to separate the layers, separate the organic layer, and wash the organic phase with saturated sodium chloride aqueous solution and water respectively , and then dried with anhydrous magnesium sulfate, filtered to remove magnesium sulfate, concentrated,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com