A kind of preparation method of egg yolk phosphatidylcholine for injection
A technology of egg yolk phosphatidylcholine and egg yolk lecithin, which is applied in the field of preparation of egg yolk phosphatidylcholine for injection, can solve the problems of high price, difficulty in ensuring the content, difficulty of single phosphatidylcholine, etc., and achieves easy production and operation , reduce over-reliance, and break the effect of foreign monopoly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
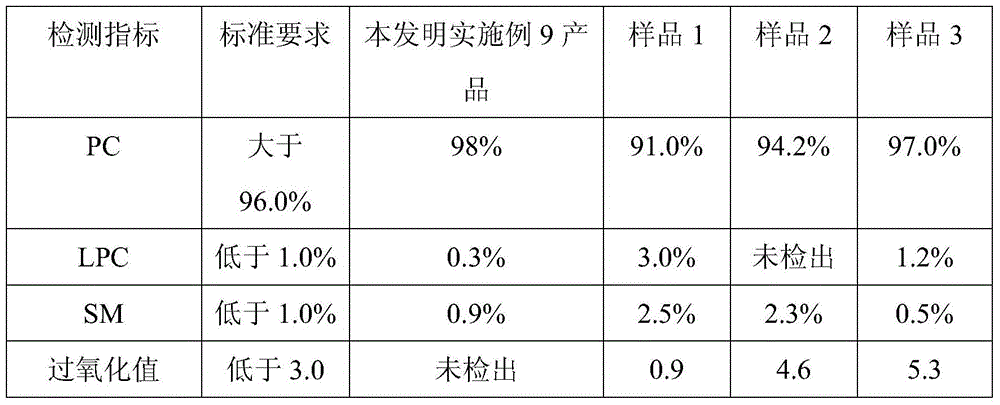
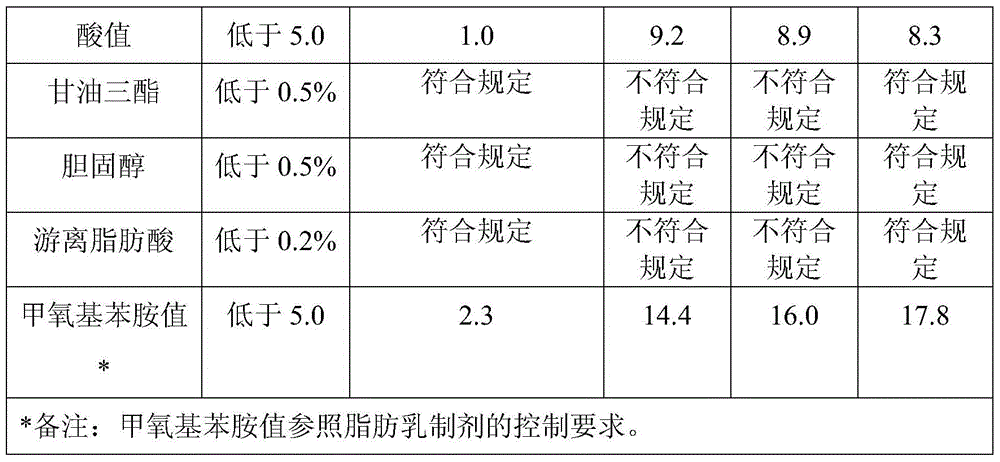
[0032] Take 1 kg of egg yolk lecithin, add chloroform to dissolve and make a raw material solution, fill 10 kg of silica gel and carry out column chromatography, wash with eluent (chloroform 77.8%, ethanol 21.8%, water 0.4%) for the first time Take off 18 liters, and then elute 60 liters with eluent (dichloromethane 44.8%, ethanol 53.7%, water 1.5%) for the second time to collect the eluate, concentrate in vacuo, and freeze-dry to obtain the phosphatidylcholine product, PC Transfer rate is 75.5%, phosphatidylcholine purity is 99.5%, lysophosphatidylcholine is not detected, sphingomyelin is 0.3%, acid value is 0.8, peroxide value is not detected, methoxyaniline value is 3.8, glycerol The contents of triester, cholesterol and free fatty acid all meet the regulations.
Embodiment 2
[0034] Get 1 kilogram of egg yolk lecithin, add dichloroethane to dissolve and be made into raw material liquid, fill 7 kilograms of silica gels and carry out column chromatography, for the first time with eluent (dichloroethane 84.0%, isopropanol 14.5%, water 1.5%) to elute 18 liters, and then elute 55 liters with eluent (chloroform 62.3%, propanol 34.7, water 3.0%) for the second time to collect the eluate, concentrate in vacuo, freeze-dry to obtain phosphatidylcholine Alkaline product, PC transfer rate 80.7%, phosphatidylcholine purity 99.1%, lysophosphatidylcholine not detected, sphingomyelin 0.5%, acid value 0.3, peroxide value not detected, methoxyaniline value 2.5, triglyceride, cholesterol, free fatty acid content all meet the regulations.
Embodiment 3
[0036]Get 5 kilograms of egg yolk lecithin, add dichloromethane to dissolve and be made into raw material liquid, fill 25 kilograms of diol-based silica gels and carry out column chromatography, for the first time with eluent (dichloromethane 89.8%, ethanol 10%, water 0.2 %) to elute 75 liters, and then elute 200 liters with eluent (chloroform 44.8%, n-butanol 52.7%, water 2.5%) for the second time to collect the eluate, concentrate in vacuo, freeze-dry to obtain phosphatidyl Choline product, PC transfer rate is 82.1%, phosphatidylcholine purity is 98.9%, lysophosphatidylcholine content is not detected, sphingomyelin is 0.3%, acid value is 0.9, peroxide value is not detected, methoxy The aniline value is 4.2, and the contents of triglyceride, cholesterol and free fatty acid all meet the regulations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com