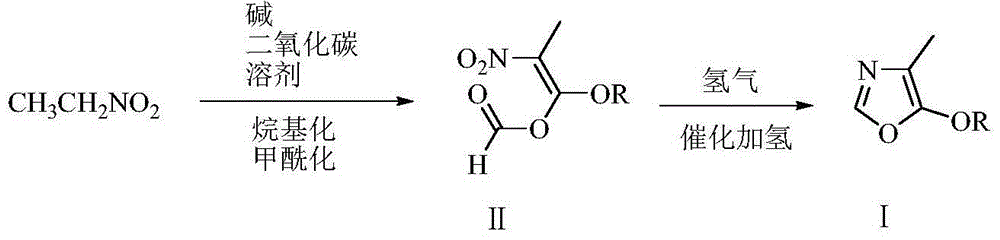
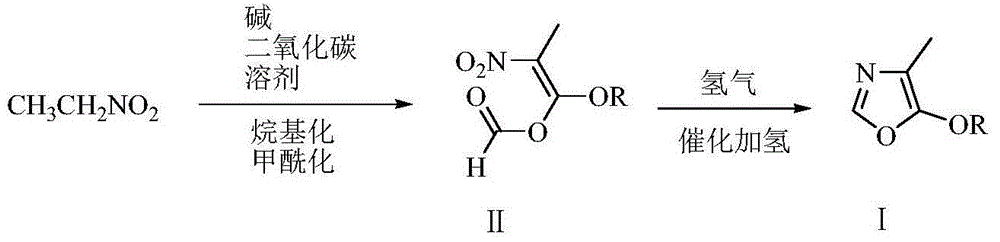
Low-cost environment-friendly preparation method of 4-methyl-5-alkoxy oxazole
A technology of alkoxyoxazole and alkoxy group, which is applied in the field of environmental protection preparation of 4-methyl-5-alkoxyoxazole, can solve problems such as unfavorable production cost control, waste water generation, and difficulty in obtaining, and achieves the reaction The effect of high atomic efficiency, short process flow and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of 4-methyl-5-methoxy oxazole
[0048] Add 220 grams (1.1 moles) of 27% sodium methylate methanol solution, 500 grams of N,N-dimethylformamide, 75.1 grams (1.0 moles) of nitroethane to the reactor equipped with tail gas absorption device , keep the inner temperature at 50-60°C under stirring, slowly feed 60 grams of carbon dioxide, and stir and react at 60-65°C for 2 hours. Cool to 10°C, add 164.0 grams (1.3 moles) of dimethyl sulfate dropwise, drop it in about 2 hours, stir and react at 10-15°C for 4 hours, then stir and react at 80-85°C for 4 hours (at this time, dimethylamine is released ). Cool to 20°C, filter, wash the filter cake with 30 grams of N,N-dimethylformamide, transfer the filtrate to a 2-liter stainless steel pressure vessel, add 20 grams of wet Pinranil nickel (water content 50%), and replace with nitrogen for 3 The hydrogenation reaction was carried out for 6 hours at 20-25° C. and 2-5 atmospheres of hydrogen. Filter, w...
Embodiment 2
[0049] Embodiment 2: the preparation of 4-methyl-5-methoxy oxazole
[0050] In the reactor, add 59.5 grams (1.1 moles) of solid sodium methylate, 500 grams of tetrahydrofuran, and 75.1 grams (1.0 moles) of nitroethane in sequence, and slowly feed 60 grams of carbon dioxide while stirring to keep the internal temperature between 50-55 ° C. And the reaction was stirred at 60 to 65°C for 2 hours. Cool to 10°C, add 164.5 grams (1.3 moles) of dimethyl sulfate dropwise, drop it in about 2 hours, stir and react at 10 to 15°C for 4 hours, then add 120.0 grams (2.0 moles) of formic acid dropwise at 20-25°C Methyl ester, dripping finished in about 3 hours, thereafter stirred and reacted for 2 hours at 20 to 25°C, filtered, and the filter cake was washed with 30 grams of tetrahydrofuran, and the filtrate was transferred to a 2-liter stainless steel pressure vessel, and 20 grams of wet pinraney nickel (water 50 %), after nitrogen replacement for 3 times, hydrogenation reaction was carrie...
Embodiment 3
[0051] Embodiment 3: the preparation of 4-methyl-5-methoxy oxazole
[0052] Add 59.5 grams (1.1 moles) of solid sodium methylate, 500 grams of tetrahydrofuran (THF), and 75.1 grams (1.0 moles) of nitroethane to the reactor in sequence, and slowly feed 60 grams of carbon dioxide while stirring to keep the internal temperature between 50 and 55°C. And the reaction was stirred at 60 to 65°C for 2 hours. Cool to 20°C, add 144.0 grams (1.6 moles) of dimethyl carbonate dropwise, drop it in about 2 hours, stir and react at 20 to 25°C for 4 hours, then add 120.0 grams (2.0 moles) of formic acid dropwise at 20 to 25°C Methyl ester, dripping finished in about 3 hours, thereafter stirred and reacted for 2 hours at 20 to 25°C, filtered, and the filter cake was washed with 30 grams of tetrahydrofuran, and the filtrate was transferred to a 2-liter stainless steel pressure vessel, and 20 grams of wet pinraney nickel (water 50 %), after nitrogen replacement for 3 times, hydrogenation reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com